Question 1
Which of the following statements apply to both glycogen and amylopectin?
1. Highly branched structure
2. Made from α–glucose monomers
3. Contains β–glucose molecules
A. 1 and 2 only
B. 2 and 3 only
C. 1 and 3 only
D. 1, 2 and 3
Easy
Mark as Complete
Mark Scheme
Question 2
Which statement is correct?
A. Triglycerides, phospholipids, and cellulose are all lipids.
B. Glucose, galactose, and fructose are all monosaccharides.
C. Amylose, sucrose, and maltose are all disaccharides.
D. Glycogen, starch, and glucose are all polysaccharides.
Easy
Mark as Complete
Mark Scheme
Question 3
A student was provided with a solution of carbohydrate. They removed two samples from the solution and performed tests on each sample, as shown.
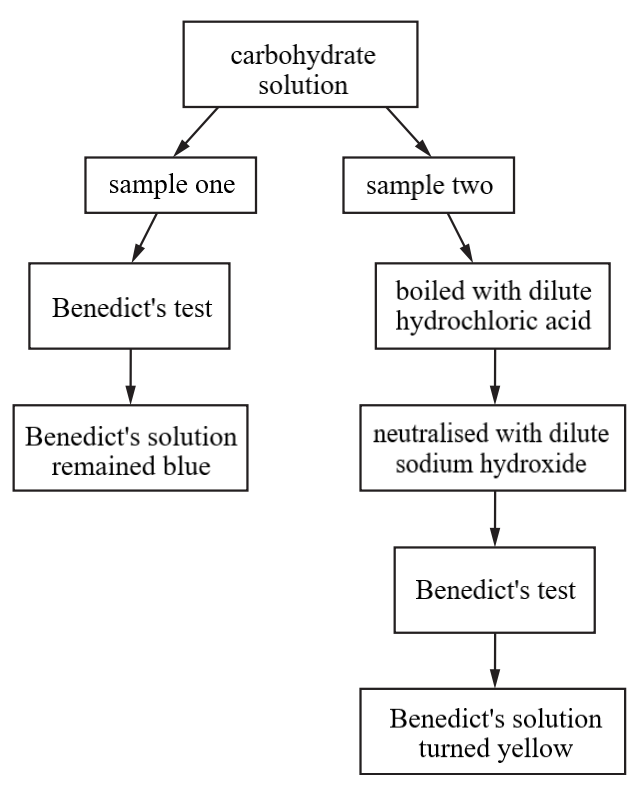
Which statement explains the results?
A. Condensation reactions occur in sample two to release reducing sugar.
B. Glycosidic bonds in a polysaccharide have been broken to release reducing sugar.
C. Sample one shows that sucrose is present in the carbohydrate solution.
D. The change in colour to a yellow solution shows that glucose is present.
Medium
Mark as Complete
Mark Scheme
Question 4
What is meant by
a. a non-reducing sugar
b. a glycosidic bond?
Easy
Mark as Complete
Mark Scheme
Question 5
Starch is a powdery material; cellulose is a strong, fibrous substance; yet both are made of glucose. What features of the cellulose molecule account for its strength?
Easy
Mark as Complete
Mark Scheme
Question 6
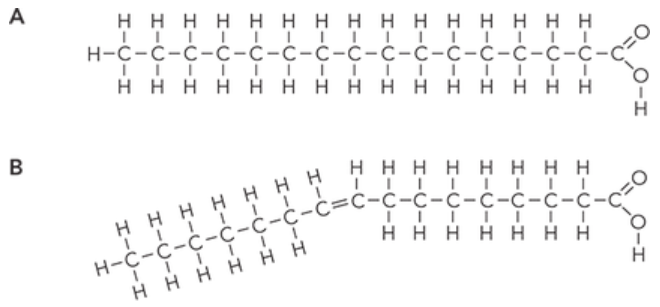
a. Which diagram from Figure 1 shows the structure of a saturated fatty acid?
 b. Figure 2 shows a triglyceride molecule.
b. Figure 2 shows a triglyceride molecule.
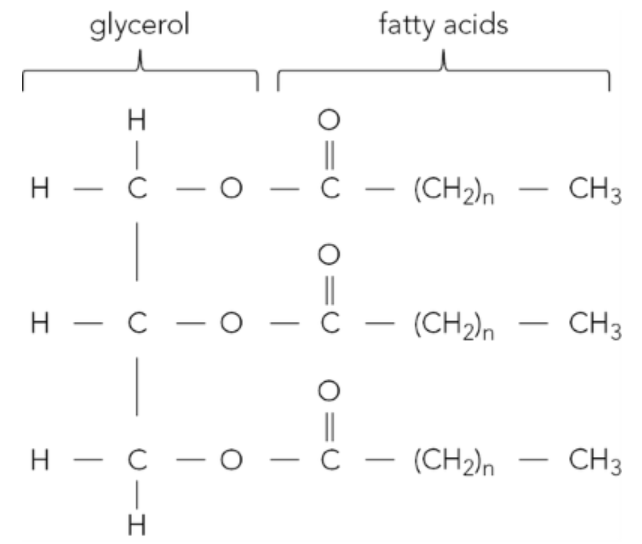
i. Identify one of the ester bonds in Figure 2 by copying the figure then drawing around an ester bond.
ii. State the name of the reaction that joined the glycerol and fatty acids together.
iii. Describe the biochemical test for a lipid.
Easy
Mark as Complete
Mark Scheme
Question 7
The figure below shows the structure of a molecule of maltose.
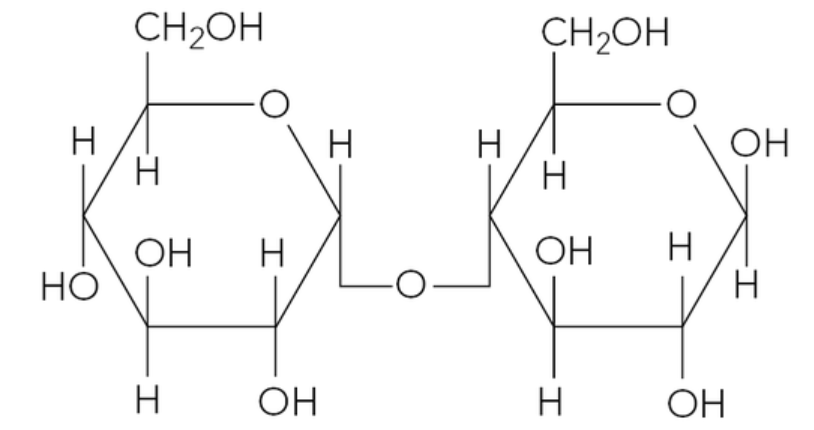
a. State the monosaccharides maltose is made of.
b. On a copy of the figure, sketch a box around the glycosidic bond.
Medium
Mark as Complete
Mark Scheme
Question 8
a. Chemical bonds are found in all biological molecules. Complete Table 1 to match up different bonds with some biological molecules.
| Hydrogen bond | Disulfide bond | Ionic bond | α(1,4) glycosidic bond | |
| found in the tertiary structure of a protein | ||||
| found in amylose | ||||
| found in cellulose | ||||
| found in the secondary structure of a protein |
Table 1.
b. A student set up a series of reactions to test the activity of the enzymes amylase and sucrase. Biochemical tests were carried out on each of the reaction mixtures after incubating at 37 °C for three hours. Table 2 shows the reactions and tests carried out.
| Tube | Contents | Iodine test | Benedict’s test | Biuret test |
| A | starch and amylase | |||
| B | starch and sucrase | |||
| C | sucrose and sucrase | |||
| D | sucrose and amylase |
Table 2.
i. Complete Table 2 by indicating which tests would give positive (+) and negative (−) results.
ii. Explain how you could carry out a biochemical test to show that a solution contained a mixture of both glucose and sucrose.
Easy
Mark as Complete
Mark Scheme
Question 9
Copy diagrams A and B.

a. Identify with labels which one represents a lipid and which a phospholipid.
b. i. For molecule A, indicate on the diagram where hydrolysis would take place if the molecule was digested.
ii. Name the products of digestion.
c. Each molecule has a head with tails attached. For molecule B, label the head to identify its chemical nature.
d. i. Which of the two molecules is water-soluble?
ii. Explain your answer to d. i.
e. State one function of each molecule.
Medium
Mark as Complete
Mark Scheme
Question 10
The figure below shows a diagram of the enzyme RNase. 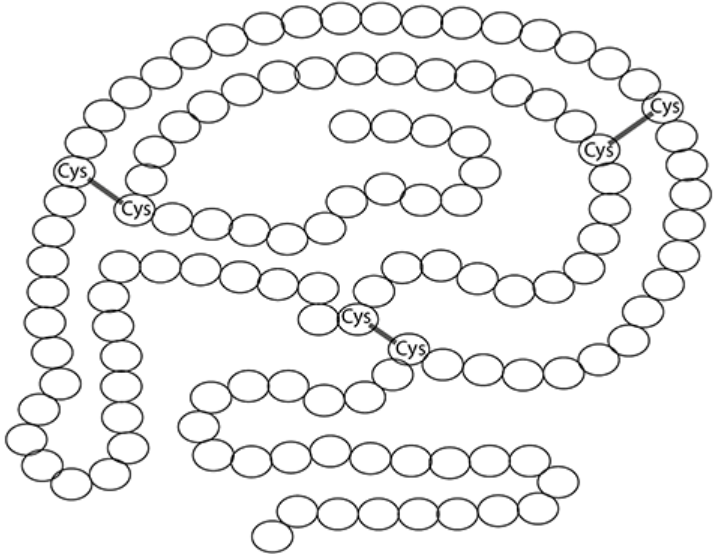
a. State the level of protein structure shown in the figure.
b. Addition of a substance called β-mercaptoethanol causes the reduction of disulfide bonds. Explain the effect this would have on RNase activity.
Hard
Mark as Complete
Mark Scheme
Question 1
Which of the following statements apply to both glycogen and amylopectin?
1. Highly branched structure
2. Made from α–glucose monomers
3. Contains β–glucose molecules
A. 1 and 2 only
B. 2 and 3 only
C. 1 and 3 only
D. 1, 2 and 3
Answer: A
1. Highly branched structure: This statement is correct.
Amylopectin is a branched chain, with branches formed by 1,6 glycosidic bonds.
Glycogen is also a branched chain. Glycogen is more highly branched or has "loads more side branches" than amylopectin. Both molecules contain 1,6 glycosidic bonds at branch points.
2. Made from α–glucose monomers: This statement is correct.
Amylopectin is a polysaccharide of alpha-glucose. Starch, which includes amylopectin, is generally formed by the condensation of α-glucose.
Glycogen is a polysaccharide of alpha-glucose. Glycogen is formed by the condensation of α-glucose.
3.Contains β–glucose molecules: This statement is incorrect.
Only cellulose is formed from the condensation of β–glucose units, while amylopectin and glycogen are made from α–glucose.
Question 2
Which statement is correct?
A. Triglycerides, phospholipids, and cellulose are all lipids.
B. Glucose, galactose, and fructose are all monosaccharides.
C. Amylose, sucrose, and maltose are all disaccharides.
D. Glycogen, starch, and glucose are all polysaccharides.
Answer: B
A. Incorrect: Triglyceride is a type of lipid. Phospholipid is also described as a type of lipid. However, cellulose is a polysaccharide, which is a type of carbohydrate, not a lipid.
B. Correct: Monosaccharides are the simplest sugars and the building blocks of carbohydrates. Glucose is a common monosaccharide. It is a hexose sugar with six carbon atoms.
Galactose is a common monosaccharide. Fructose is a common monosaccharide.
C. Incorrect: Disaccharides are formed from two monosaccharides by condensation reactions. Sucrose is a disaccharide formed from glucose and fructose. Maltose is a disaccharide formed from two glucose molecules. However, amylose is a component of starch and is a polysaccharide.
D. Incorrect: Glycogen is a polysaccharide. Starch is a polysaccharide. However, glucose is a monosaccharide, which is a single sugar unit, the monomer of polysaccharides.
Question 3
A student was provided with a solution of carbohydrate. They removed two samples from the solution and performed tests on each sample, as shown.

Which statement explains the results?
A. Condensation reactions occur in sample two to release reducing sugar.
B. Glycosidic bonds in a polysaccharide have been broken to release reducing sugar.
C. Sample one shows that sucrose is present in the carbohydrate solution.
D. The change in colour to a yellow solution shows that glucose is present.
Answer: B
• Sample one (Benedict’s test): Benedict’s solution remained blue. This indicates the absence of reducing sugars in the original carbohydrate solution.
• Sample two (boiled with dilute hydrochloric acid, neutralised, then Benedict’s test): Benedict’s solution turned yellow. This procedure is a test for non-reducing sugars or hydrolysable polysaccharides. A positive result after this treatment indicates that the original carbohydrate was broken down into reducing sugars by the acid hydrolysis.
A. Incorrect: Condensation reactions are chemical reactions that join two molecules together to form a larger molecule, usually with the removal of a water molecule. The process in Sample two involves boiling with dilute acid, which breaks down larger carbohydrates into smaller molecules. Breaking chemical bonds in larger molecules to release smaller ones is called hydrolysis, and it involves the addition of water. Therefore, reducing sugars are released by hydrolysis, not condensation.
B. Correct: Polysaccharides are large carbohydrate molecules made up of many monosaccharides linked together by glycosidic bonds. Polysaccharides themselves are generally not reducing sugars. The treatment in Sample two causes acid hydrolysis, which specifically breaks these glycosidic bonds. When glycosidic bonds in polysaccharides are broken, they release their constituent monosaccharides or smaller oligosaccharides, many of which are reducing sugars (e.g., glucose from starch or glycogen hydrolysis). Since Sample one showed no reducing sugar initially, and Sample two showed reducing sugar after hydrolysis, the original carbohydrate could have been a polysaccharide that was broken down into reducing sugars.
C. Incorrect: Sample one involved a direct Benedict's test, and the solution remained blue. This result indicates the absence of reducing sugars. Sucrose is a non-reducing sugar. A negative Benedict's test is consistent with the presence of sucrose, as sucrose would not give a positive direct test. However, the negative result in Sample one only rules out the presence of significant amounts of reducing sugars; it does not specifically show that sucrose is present. The original carbohydrate could be any non-reducing sugar or hydrolysable polysaccharide.
D. Incorrect: The change in colour of Benedict's solution to yellow is indeed a positive test for the presence of reducing sugars. Glucose is a common monosaccharide and is a reducing sugar. It is highly probable that glucose (among other possible reducing sugars) was released during the hydrolysis in Sample two, but we cannot confirm it's glucose specifically without further tests.
However, the statement only describes what the yellow colour indicates after the treatment in Sample Two; it does not explain why the reducing sugar appeared only after hydrolysis, which is crucial information provided by comparing the results of Sample one and Sample two.
Question 4
What is meant by
a. a non-reducing sugar
b. a glycosidic bond?
a. A non-reducing sugar
The classification of sugars as reducing or non-reducing depends on their ability to donate electrons.
A non-reducing sugar cannot donate electrons and therefore cannot act as a reducing agent, meaning it does not reduce copper(II) ions. Non-reducing sugars typically do not have a free aldehyde or ketone group available to react with Benedict's reagent. When heated with Benedict's solution, the blue color remains.
A common example of a non-reducing sugar mentioned in the sources is sucrose. All monosaccharides are reducing sugars, as are some disaccharides like maltose and lactose.
To test for a non-reducing sugar like sucrose, it must first be broken down into its constituent monosaccharides. This is achieved by a hydrolysis reaction, usually by heating the sample with dilute hydrochloric acid. After hydrolysis, the solution needs to be neutralised, typically with sodium hydrogencarbonate. Following neutralisation, the Benedict's test is carried out as for a reducing sugar. If a colored precipitate forms (such as green, yellow, orange, or brick-red), it indicates that a non-reducing sugar was present in the original sample.
b. A glycosidic bond
A glycosidic bond is a strong covalent bond that joins monosaccharide molecules together. It is often described as a C-O-C link between two sugar molecules.
Glycosidic bonds are formed by condensation reactions between two hydroxyl (-OH) groups on adjacent monosaccharides. During this reaction, a molecule of water is released.
Examples of glycosidic bonds include the link between two alpha-glucose molecules to form maltose, between alpha-glucose and fructose to form sucrose, or bsetween glucose and galactose to form lactose. Glycosidic bonds also link many monosaccharides to form larger polysaccharides like starch, glycogen, and cellulose.
The breakage of glycosidic bonds occurs during hydrolysis reactions, which involve the addition of a molecule of water and are catalysed by enzymes. This is the process that breaks down disaccharides and polysaccharides into their constituent monosaccharides, for example, during digestion.
Question 5
Starch is a powdery material; cellulose is a strong, fibrous substance; yet both are made of glucose. What features of the cellulose molecule account for its strength?
Both starch and cellulose are polysaccharides, meaning they are polymers built from many glucose molecules joined together. However, they have very different structures that account for why starch is powdery and cellulose is strong and fibrous.
Starch is made from alpha-glucose molecules. Cellulose is made from beta-glucose molecules.
In starch, the alpha-glucose units are joined by glycosidic bonds, primarily 1-4 glycosidic bonds. Starch is a mixture of amylose and amylopectin. Amylose is an unbranched chain that coils into a helix, making it compact. Amylopectin is branched, with 1-6 glycosidic bonds forming the branches.
In cellulose, the beta-glucose units are also joined by 1-4 glycosidic bonds. However, because of the beta configuration of carbon 1, successive glucose molecules are rotated 180° relative to each other to form the bond. This results in long, straight, unbranched chains.
The coiled and branched structures of starch molecules (amylose and amylopectin) bundle together to form compact starch grains. Starch is described as a powdery material.
The straight cellulose chains can lie parallel to each other, and a large number of hydrogen bonds form between the hydroxyl groups on adjacent chains. These bundles of hydrogen-bonded chains are called microfibrils, which are then held together in fibres. These strong fibres give cellulose its high tensile strength, making it a key structural component of plant cell walls. The arrangement of fibres in different layers further increases the strength of the cell wall.
Therefore, the different orientation of the glycosidic bonds due to the use of beta-glucose instead of alpha-glucose leads to straight cellulose chains, which form extensive hydrogen bonds with neighboring chains, creating strong, fibrous microfibrils and fibers. Starch, made of alpha-glucose, forms coiled and branched molecules that pack into powdery grains instead.
Question 6
a. Which diagram from Figure 1 shows the structure of a saturated fatty acid?
 b. Figure 2 shows a triglyceride molecule.
b. Figure 2 shows a triglyceride molecule.

i. Identify one of the ester bonds in Figure 2 by copying the figure then drawing around an ester bond.
ii. State the name of the reaction that joined the glycerol and fatty acids together.
iii. Describe the biochemical test for a lipid.
a. Diagram A from Figure 1 is a saturated fatty acid. A saturated fatty acid is a type of fatty acid that does not have any double bonds between the carbon atoms in its hydrocarbon tail (forms a straight chain). This means that every carbon atom in the hydrocarbon tail is attached to the maximum possible number of hydrogen atoms, hence it is saturated with hydrogen.
b.
i.
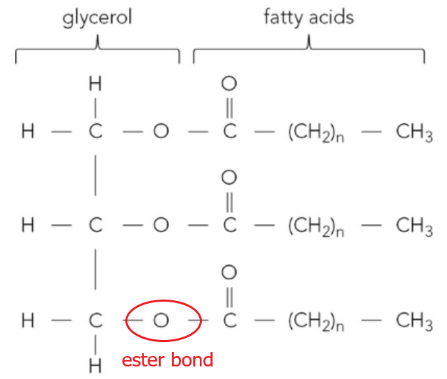
ii. The reaction that joined the glycerol and fatty acids together is condensation. This reaction occurs between the hydroxyl (-OH) groups of glycerol and the carboxyl (-COOH) groups of the fatty acids, which form ester bonds.
iii. The biochemical test for a lipid is called the emulsion test.
If the substance to be tested is a solid, you may need to prepare a solution or suspension of it, perhaps by crushing it with water and then filtering, or by crushing it directly with ethanol.
The test substance is shaken vigorously with ethanol, specifically described as absolute ethanol, for about a minute. This dissolves any lipids present in the substance. The ethanol solution is then poured into water.
If lipids are present, a cloudy white suspension will form. This is described as a milky emulsion. The more lipid present, the more noticeable or obvious the milky colour will be. If no lipid is present, the solution will remain clear or transparent.
The test works because lipids are insoluble in water (hydrophobic) but are soluble in organic solvents like ethanol. When the ethanol containing the dissolved lipid is mixed with water, the lipid comes out of solution and disperses in the water as tiny droplets, creating the characteristic milky emulsion.
Question 7
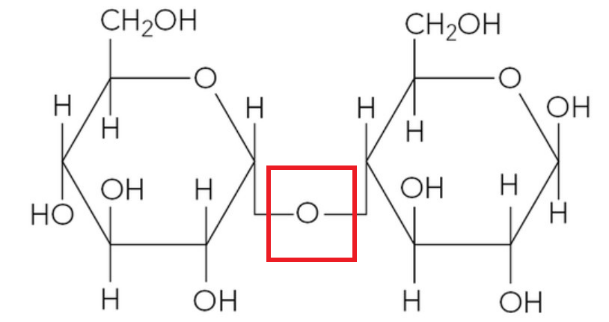
The figure below shows the structure of a molecule of maltose.

a. State the monosaccharides maltose is made of.
b. On a copy of the figure, sketch a box around the glycosidic bond.
a. Maltose is a disaccharide formed from two glucose molecules. Specifically, it is formed from two alpha-glucose molecules joined together.
b. The glycosidic bond is the oxygen atom that links the two glucose rings together. It is a covalent bond formed during a condensation reaction. In maltose, this bond typically links C1 of one glucose molecule to C4 of the other glucose molecule.
Question 8
a. Chemical bonds are found in all biological molecules. Complete Table 1 to match up different bonds with some biological molecules.
| Hydrogen bond | Disulfide bond | Ionic bond | α(1,4) glycosidic bond | |
| found in the tertiary structure of a protein | ||||
| found in amylose | ||||
| found in cellulose | ||||
| found in the secondary structure of a protein |
Table 1.
b. A student set up a series of reactions to test the activity of the enzymes amylase and sucrase. Biochemical tests were carried out on each of the reaction mixtures after incubating at 37 °C for three hours. Table 2 shows the reactions and tests carried out.
| Tube | Contents | Iodine test | Benedict’s test | Biuret test |
| A | starch and amylase | |||
| B | starch and sucrase | |||
| C | sucrose and sucrase | |||
| D | sucrose and amylase |
Table 2.
i. Complete Table 2 by indicating which tests would give positive (+) and negative (−) results.
ii. Explain how you could carry out a biochemical test to show that a solution contained a mixture of both glucose and sucrose.
a.
| Hydrogen bond | Disulfide bond | Ionic bond | α(1,4) glycosidic bond | |
| found in the tertiary structure of a protein | ✗ | ✗ | ✗ | |
| found in amylose | ✗ | |||
| found in cellulose | ✗ | |||
| found in the secondary structure of a protein | ✗ |
b. i.
| Tube | Contents | Iodine test | Benedict’s test | Biuret test |
| A | starch and amylase | – | + | + |
| B | starch and sucrase | + | – | + |
| C | sucrose and sucrase | – | + | + |
| D | sucrose and amylase | – | – | + |
Tube A – starch and amylase: Amylase is an enzyme that catalyses the breakdown of starch to maltose. Maltose is a reducing sugar. After incubating, it is expected that the starch would be hydrolysed into maltose.
Tube B – starch and sucrase: Sucrase is an enzyme that breaks down sucrose into glucose and fructose. It does not act on starch. Therefore, starch remains in the tube.
Tube C – sucrose and sucrase: Sucrase catalyses the breakdown of sucrose into glucose and fructose. Glucose and fructose are monosaccharides and are reducing sugars. After incubating, it is expected that the sucrose would be hydrolysed into glucose and fructose.
Tube D – Sucrose and amylase: Amylase breaks down starch, it does not act on sucrose. Sucrose remains in the tube.
ii.
To show that a solution contains both glucose (a reducing sugar) and sucrose (a non-reducing sugar), perform the following series of tests:
• Test for reducing sugars (glucose) in the original solution
Take a sample of the solution. Add Benedict's reagent (blue). Heat the mixture in a water bath brought to the boil. If glucose is present, a coloured precipitate (green, yellow, orange, or brick red) will form. Since glucose is expected to be present in this mixture, this test will yield a positive result.
• Test for non-reducing sugars (sucrose) in a separate sample
Take a new sample of the test solution (not the one already tested with Benedict's). Add dilute hydrochloric acid. Carefully heat the mixture in a water bath that has been brought to the boil. This step hydrolyses the sucrose into its constituent monosaccharides, glucose and fructose, both of which are reducing sugars.
• Neutralise the acid
After heating, cool the solution. Neutralise the acid by adding sodium hydrogencarbonate until it stops fizzing. The solution should now be slightly alkaline for the Benedict's test to work effectively.
• Test for reducing sugars after hydrolysis
Finally, add Benedict's reagent to this neutralised sample. Heat it in a water bath that has been brought to the boil. Since the sucrose (if present) has been broken down into glucose and fructose, and glucose was already present, this test should now show a positive result.
• Interpret the results
A positive result in the first test indicates the presence of reducing sugar like glucose. If the second test (after hydrolysis) gives a more intense coloured precipitate or a higher level of precipitate compared to the first test, this indicates that more reducing sugars were produced by hydrolysing a non-reducing sugar like sucrose.
If the first test was positive, and the second test is still positive (as expected because glucose was present), a quantitative or semi-quantitative comparison of the colour intensity or precipitate amount is needed to confirm the presence of sucrose in addition to the initial reducing sugar. Note that using the same volume of the original solution for both tests is important for comparison.
Question 9
Copy diagrams A and B.

a. Identify with labels which one represents a lipid and which a phospholipid.
b. i. For molecule A, indicate on the diagram where hydrolysis would take place if the molecule was digested.
ii. Name the products of digestion.
c. Each molecule has a head with tails attached. For molecule B, label the head to identify its chemical nature.
d. i. Which of the two molecules is water-soluble?
ii. Explain your answer to d. i.
e. State one function of each molecule.
a.
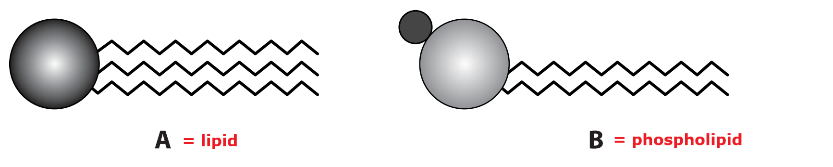
Diagram A (a head and three tails) represents a lipid, specifically a triglyceride. Triglycerides are formed from one molecule of glycerol and three fatty acid molecules joined by ester bonds. They have long hydrocarbon tails that are hydrophobic, making them insoluble in water.
Diagram B (a head and two tails) represents a phospholipid. Phospholipids are similar to triglycerides, but one fatty acid molecule is replaced by a phosphate group. This results in a structure with a hydrophilic (water-attracting) head and hydrophobic (water-repelling) tails. The hydrophilic nature of the head is due to the presence of the phosphate group, which is ionised or polar.
b.
i. Hydrolysis occurs at the ester bonds between the glycerol head and each fatty acid tail. Indicate the junction between the round head and the zigzag tails for all three tails on molecule A.
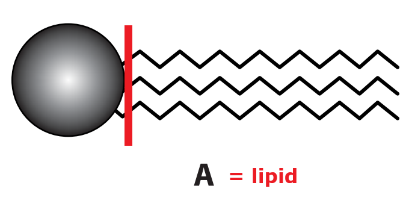
ii. After the hydrolysis of a triglyceride, the products are fatty acids and glycerol.
c. The head of a phospholipid contains a phosphate group, which is polar and hydrophilic.
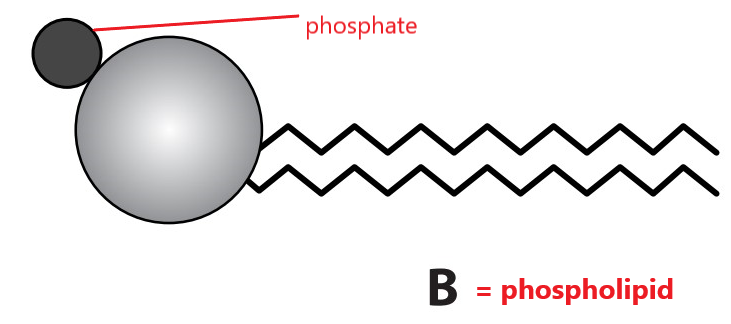
d.
i.The molecule B (phospholipid) is water-soluble.
ii. Phospholipid is water-soluble because the phosphate head contains a phosphate group, which is polar and hydrophilic, so it interacts well with water.
Note that while triglycerides (molecule A) are generally insoluble due to their hydrophobic tails, the phospholipid (molecule B) has a hydrophilic phosphate head that makes it water-soluble.
e.
Molecule A: Lipid (Triglyceride)
Molecule B: Phospholipid
Question 10
The figure below shows a diagram of the enzyme RNase. 
a. State the level of protein structure shown in the figure.
b. Addition of a substance called β-mercaptoethanol causes the reduction of disulfide bonds. Explain the effect this would have on RNase activity.
a. The figure shows the tertiary (3°) structure of the enzyme RNase. The tertiary structure is the complex three-dimensional shape formed by the further folding and coiling of the polypeptide chain, held in place by various bonds.
b. RNase is an enzyme, and enzymes are proteins. The activity of an enzyme is highly dependent on its specific three-dimensional shape, which is its tertiary structure. Disulfide bonds, formed between cysteine amino acids, are strong covalent bonds that are crucial in maintaining the tertiary structure of proteins.
When a substance like β-mercaptoethanol causes the reduction of disulfide bonds, these bonds break. Breaking these bonds disrupts the forces holding the protein in its specific folded shape. This leads to changes in the tertiary structure, or denaturation of the enzyme.
The enzyme's active site, where the substrate binds, is formed by the folding of the polypeptide chain (its tertiary structure). When the tertiary structure changes, the active site changes shape. Since the active site's shape is altered, the substrate molecule can no longer fit into it properly. This prevents the formation of the enzyme-substrate complex, which is necessary for the enzyme to catalyse the reaction efficiently.
Therefore, the reduction of disulfide bonds by β-mercaptoethanol causes the enzyme to lose its functional shape, resulting in a significant decrease or complete loss of RNase activity.
Question 1
Which of the following statements apply to both glycogen and amylopectin?
1. Highly branched structure
2. Made from α–glucose monomers
3. Contains β–glucose molecules
A. 1 and 2 only
B. 2 and 3 only
C. 1 and 3 only
D. 1, 2 and 3
Question 2
Which statement is correct?
A. Triglycerides, phospholipids, and cellulose are all lipids.
B. Glucose, galactose, and fructose are all monosaccharides.
C. Amylose, sucrose, and maltose are all disaccharides.
D. Glycogen, starch, and glucose are all polysaccharides.
Question 3
A student was provided with a solution of carbohydrate. They removed two samples from the solution and performed tests on each sample, as shown.

Which statement explains the results?
A. Condensation reactions occur in sample two to release reducing sugar.
B. Glycosidic bonds in a polysaccharide have been broken to release reducing sugar.
C. Sample one shows that sucrose is present in the carbohydrate solution.
D. The change in colour to a yellow solution shows that glucose is present.
Question 4
What is meant by
a. a non-reducing sugar
b. a glycosidic bond?
Question 5
Starch is a powdery material; cellulose is a strong, fibrous substance; yet both are made of glucose. What features of the cellulose molecule account for its strength?
Question 6
a. Which diagram from Figure 1 shows the structure of a saturated fatty acid?
 b. Figure 2 shows a triglyceride molecule.
b. Figure 2 shows a triglyceride molecule.

i. Identify one of the ester bonds in Figure 2 by copying the figure then drawing around an ester bond.
ii. State the name of the reaction that joined the glycerol and fatty acids together.
iii. Describe the biochemical test for a lipid.
Question 7
The figure below shows the structure of a molecule of maltose.

a. State the monosaccharides maltose is made of.
b. On a copy of the figure, sketch a box around the glycosidic bond.
Question 8
a. Chemical bonds are found in all biological molecules. Complete Table 1 to match up different bonds with some biological molecules.
| Hydrogen bond | Disulfide bond | Ionic bond | α(1,4) glycosidic bond | |
| found in the tertiary structure of a protein | ||||
| found in amylose | ||||
| found in cellulose | ||||
| found in the secondary structure of a protein |
Table 1.
b. A student set up a series of reactions to test the activity of the enzymes amylase and sucrase. Biochemical tests were carried out on each of the reaction mixtures after incubating at 37 °C for three hours. Table 2 shows the reactions and tests carried out.
| Tube | Contents | Iodine test | Benedict’s test | Biuret test |
| A | starch and amylase | |||
| B | starch and sucrase | |||
| C | sucrose and sucrase | |||
| D | sucrose and amylase |
Table 2.
i. Complete Table 2 by indicating which tests would give positive (+) and negative (−) results.
ii. Explain how you could carry out a biochemical test to show that a solution contained a mixture of both glucose and sucrose.
Question 9
Copy diagrams A and B.

a. Identify with labels which one represents a lipid and which a phospholipid.
b. i. For molecule A, indicate on the diagram where hydrolysis would take place if the molecule was digested.
ii. Name the products of digestion.
c. Each molecule has a head with tails attached. For molecule B, label the head to identify its chemical nature.
d. i. Which of the two molecules is water-soluble?
ii. Explain your answer to d. i.
e. State one function of each molecule.
Question 10
The figure below shows a diagram of the enzyme RNase. 
a. State the level of protein structure shown in the figure.
b. Addition of a substance called β-mercaptoethanol causes the reduction of disulfide bonds. Explain the effect this would have on RNase activity.