Question 1
An isotope of boron decays to beryllium by β⁺ emission.
Which particle is emitted in addition to the β⁺ particle?
A. Antineutrino.
B. Electron.
C. Neutrino.
D. Neutron.
Easy
Mark as Complete
Mark Scheme
Question 2
Which statement about radioactive decay is correct?
A. Neutrinos are always emitted during α-decay.
B. The α-particles emitted from a radioactive sample have a continuous range of kinetic energies.
C. The β⁻ particles emitted from a radioactive sample have a continuous range of kinetic energies.
D. The proton number of a nucleus decreases by four when it undergoes α-decay.
Easy
Mark as Complete
Mark Scheme
Question 3
Nuclide X with proton number Z undergoes β⁺ decay to form nuclide Y.
The decay may be represented by the equation shown:
`X rightarrow Y + beta^++ W`"
What is the proton number of Y and which particle is represented by the symbol W?
| Proton number of Y | Particle represented by W | |
|---|---|---|
| A | Z − 1 | antineutrino |
| B | Z − 1 | neutrino |
| C | Z + 1 | antineutrino |
| D | Z + 1 | neutrino |
Easy
Mark as Complete
Mark Scheme
Question 4
The unstable nuclide 21884 decays through a sequence of emissions of α and β⁻ particles to form the stable nuclide 21083.
How many α and β⁻ particles are emitted during this decay process?
| α-particles | β⁻ particles | |
|---|---|---|
| A | 1 | 1 |
| B | 2 | 1 |
| C | 2 | 3 |
| D | 3 | 2 |
Easy
Mark as Complete
Mark Scheme
Question 5
What are isotopes?
A. Nuclei of different elements with the same number of neutrons.
B. Nuclei of different elements with the same number of nucleons.
C. Nuclei of the same element with different numbers of neutrons.
D. Nuclei of the same element with different numbers of protons.
Easy
Mark as Complete
Mark Scheme
Question 6
In an experiment on α-particle scattering, α-particles are directed at a thin gold foil. Most of the α-particles pass straight through the foil or are deflected by a small angle. A small number of α-particles are deflected by a large angle.
Which statement cannot be deduced from this experiment?
A. Atoms are mostly empty space.
B. Most of the mass of an atom is concentrated in the nucleus.
C. The nucleus of an atom contains protons.
D. The nucleus of an atom is small compared to the size of an atom.
Easy
Mark as Complete
Mark Scheme
Question 7
Which two particles have opposite charges?
A. Alpha-particle and helium nucleus
B. Antiproton and beta-plus particle
C. Beta-minus particle and electron
D. Positron and proton
Easy
Mark as Complete
Mark Scheme
Question 8
What is a conclusion from the alpha-particle scattering experiment?
A. Protons and electrons have equal but opposite charges.
B. Protons have a much larger mass than electrons.
C. The nucleus contains most of the mass of the atom.
D. The nucleus of an atom contains protons and neutrons.
Medium
Mark as Complete
Mark Scheme
Question 9
a. Compare an α-particle with a β⁺ particle in terms of their masses and charges.
b. Nucleus P undergoes α-decay to form nucleus Q. Nucleus Q then undergoes a further decay to form nucleus R. The proton and nucleon numbers of P and R are shown in figure.
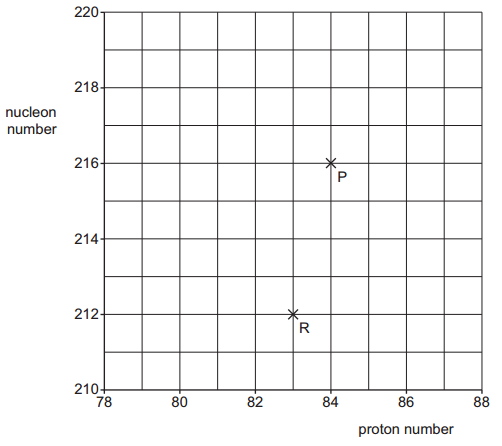 i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
ii. State the names of the particles emitted as Q decays to form R.
c. Before the α-decay, P is travelling at a constant velocity. After the decay, Q has a velocity of 1.3 × 105 m.s−1 at an angle of 68∘ to the original path of P.
The α-particle has a velocity of 150 × 105 m.s−1 at an angle of θ to the original path of P, as shown in figure.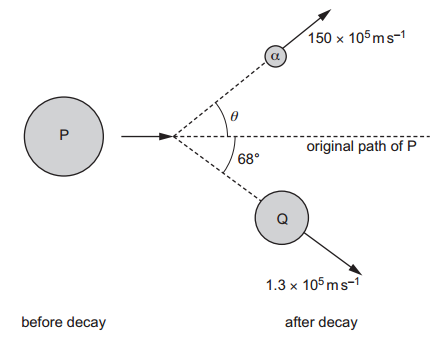 i. Use the principle of conservation of momentum to determine θ.
i. Use the principle of conservation of momentum to determine θ.
ii.a Calculate the kinetic energy of the α‑particle.
Hard
Mark as Complete
Mark Scheme
Question 10
a. One of the results of the α-particle scattering experiment is that a very small minority of the α-particles are scattered through angles greater than 90°.
State what may be inferred about the structure of the atom from this result.
b. An α-particle is made up of other particles. One of these particles is a proton.
State and explain whether a proton is a fundamental particle.
c. A radioactive source produces a beam of α-particles in a vacuum. The average current produced by the beam is 6.9 × 10−9 A.
Calculate the average number of α-particles passing a fixed point in the beam in a time of 1.0 minute.
d. The α-particles in the vacuum in (c) enter a uniform electric field. The α-particles enter the field with their velocity in the same direction as the field.
State and explain whether the magnitude of the acceleration of an α-particle due to the field decreases, increases or stays constant as the α-particle moves through the field.
e. A nucleus X is an isotope of a nucleus Y. The mass of nucleus X is greater than that of Y.
Both of the nuclei are in the same uniform electric field.
State and explain whether the magnitude of the electric force acting on nucleus X is greater than, less than or the same as that acting on nucleus Y.
Hard
Mark as Complete
Mark Scheme
Question 1
An isotope of boron decays to beryllium by β⁺ emission.
Which particle is emitted in addition to the β⁺ particle?
A. Antineutrino.
B. Electron.
C. Neutrino.
D. Neutron.
Answer: C
In β⁺ decay, a proton in the nucleus forms a neutron, a positive electron (positron), and a neutrino.
So, during β⁺ emission, a neutrino is always emitted along with the positron. This helps conserve lepton number and energy.
Question 2
Which statement about radioactive decay is correct?
A. Neutrinos are always emitted during α-decay.
B. The α-particles emitted from a radioactive sample have a continuous range of kinetic energies.
C. The β⁻ particles emitted from a radioactive sample have a continuous range of kinetic energies.
D. The proton number of a nucleus decreases by four when it undergoes α-decay.
Answer: C
A. Incorrect: Neutrinos and antineutrinos are emitted in β-decay, not in α-decay.
B. Incorrrect: The α-particles emitted from a particular radioactive nuclide all have the same kinetic energy.
C. Correct: The β-particles from a particular nuclide have a continuous range of energies, from zero to the maximum energy available... because neutrinos or antineutrinos are emitted in β-decay.
D. Incorrect: In α-decay, the proton number decreases by two and the nucleon number decreases by four.
Question 3
Nuclide X with proton number Z undergoes β⁺ decay to form nuclide Y.
The decay may be represented by the equation shown:
`X rightarrow Y + beta^++ W`"
What is the proton number of Y and which particle is represented by the symbol W?
| Proton number of Y | Particle represented by W | |
|---|---|---|
| A | Z − 1 | antineutrino |
| B | Z − 1 | neutrino |
| C | Z + 1 | antineutrino |
| D | Z + 1 | neutrino |
Answer: B
A. Incorrect: Proton number is correct (Z − 1) but antineutrino is wrong - should be a neutrino.
B. Correct: In β⁺ decay, a proton turns into a neutron, emitting: a positron and a neutrino.
So: Proton number decreases by 1 and the emitted particle (W) is a neutrino.
C. Incorrect: Proton number is wrong (Z + 1 - should decrease). Antineutrino is also incorrect.
D. Incorrect: Proton number is wrong (Z + 1). Neutrino is correct - but both must be right.
Question 4
The unstable nuclide 21884 decays through a sequence of emissions of α and β⁻ particles to form the stable nuclide 21083.
How many α and β⁻ particles are emitted during this decay process?
| α-particles | β⁻ particles | |
|---|---|---|
| A | 1 | 1 |
| B | 2 | 1 |
| C | 2 | 3 |
| D | 3 | 2 |
Answer: C
Conservation of nucleon number (A):
`A_("final") = A_("initial") - 4a => 210 = 218 - 4a => 4a = 8 => a = 2`
Conservation of proton (Z):
`Z_("final") = Z_("initial") - 2a + b => 83 = 84 - 2(2) + b => 83 = 84 - 4 + b => b = 3`
α-particles: 2
β⁻ particles: 3
Question 5
What are isotopes?
A. Nuclei of different elements with the same number of neutrons.
B. Nuclei of different elements with the same number of nucleons.
C. Nuclei of the same element with different numbers of neutrons.
D. Nuclei of the same element with different numbers of protons.
Answer: C
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons
Question 6
In an experiment on α-particle scattering, α-particles are directed at a thin gold foil. Most of the α-particles pass straight through the foil or are deflected by a small angle. A small number of α-particles are deflected by a large angle.
Which statement cannot be deduced from this experiment?
A. Atoms are mostly empty space.
B. Most of the mass of an atom is concentrated in the nucleus.
C. The nucleus of an atom contains protons.
D. The nucleus of an atom is small compared to the size of an atom.
Answer: C
A. Incorrect: Most α-particles pass through → atom is mostly empty space.
B. Incorrect: Large-angle deflections imply α-particles hit a massive, concentrated core → the nucleus.
C. Correct:
The experiment only revealed that the nucleus is positively charged, not what particles are inside.
At the time, protons had not yet been definitively identified as constituents of the nucleus. The experiment could not distinguish whether the nucleus contained protons, positrons, or other positive particles.
D. Incorrect: Very few deflections occur → nucleus must be small relative to atom size.
Question 7
Which two particles have opposite charges?
A. Alpha-particle and helium nucleus
B. Antiproton and beta-plus particle
C. Beta-minus particle and electron
D. Positron and proton
Answer: B
Antiproton = antiparticle of the proton → charge = −1e
Beta-plus particle = positron → charge = +1e
Question 8
What is a conclusion from the alpha-particle scattering experiment?
A. Protons and electrons have equal but opposite charges.
B. Protons have a much larger mass than electrons.
C. The nucleus contains most of the mass of the atom.
D. The nucleus of an atom contains protons and neutrons.
Answer: C
A. Incorrect: It’s based on charge conservation and later experiments, not Rutherford scattering.
B. Incorrect: While protons are much heavier than electrons, this fact comes from mass measurements, not the alpha-scattering experiment.
C. Correct:
In the Rutherford scattering experiment, most alpha particles passed straight through the gold foil, while a small number were deflected by large angles. This led to two major conclusions:
1.The atom is mostly empty space (since most particles went through).
2. There is a small, dense, massive central core (the nucleus) that causes strong deflection → the nucleus contains most of the atom's mass.
D. Incorrect: Neutrons were not discovered at the time of Rutherford’s experiment (discovered later in 1932 by James Chadwick), so the experiment could not reveal their presence.
Question 9
a. Compare an α-particle with a β⁺ particle in terms of their masses and charges.
b. Nucleus P undergoes α-decay to form nucleus Q. Nucleus Q then undergoes a further decay to form nucleus R. The proton and nucleon numbers of P and R are shown in figure.
 i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
ii. State the names of the particles emitted as Q decays to form R.
c. Before the α-decay, P is travelling at a constant velocity. After the decay, Q has a velocity of 1.3 × 105 m.s−1 at an angle of 68∘ to the original path of P.
The α-particle has a velocity of 150 × 105 m.s−1 at an angle of θ to the original path of P, as shown in figure. i. Use the principle of conservation of momentum to determine θ.
i. Use the principle of conservation of momentum to determine θ.
ii.a Calculate the kinetic energy of the α‑particle.
a. The mass of an α-particle is much greater than that of a β⁺ particle.
An α-particle is a helium nucleus: contains 2 protons and 2 neutrons (mass ≈ 4u).
A β⁺ particle is a positron: same mass as an electron (≈ 0.00055u).
Both particles are positively charged.
The charge on an α-particle is +2e, while the β⁺ particle has a charge of +e.
b. i. P → Q by α-decay:
So, Q must be:
Proton number = 84 − 2 = 82.
Nucleon number = 216 − 4 = 212.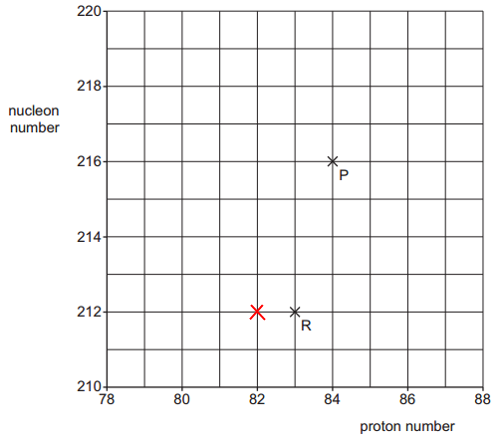 ii. Particles emitted:
ii. Particles emitted:
β⁻ particle (electron).
Electron antineutrino.
c.
i. Vertical (y-axis) components of momentum:
`212u xx 1.3 xx 10^5 xx sin(68^@) = 4u xx 150 xx 10^5 xx sin(theta)`
`sin(theta) = (212 xx 1.3 xx sin(68^@)) / (4 xx 150) = (276 xx 0.927) / 600 = 0.426 => theta = sin^-1(0.426)=25^@`
ii. `E = (1/2)mv^2= (1/2) xx 4 xx 1.66xx10^-27 xx (150 xx 10^5)^2 = 7.5 xx 10^-13 " J"`
Question 10
a. One of the results of the α-particle scattering experiment is that a very small minority of the α-particles are scattered through angles greater than 90°.
State what may be inferred about the structure of the atom from this result.
b. An α-particle is made up of other particles. One of these particles is a proton.
State and explain whether a proton is a fundamental particle.
c. A radioactive source produces a beam of α-particles in a vacuum. The average current produced by the beam is 6.9 × 10−9 A.
Calculate the average number of α-particles passing a fixed point in the beam in a time of 1.0 minute.
d. The α-particles in the vacuum in (c) enter a uniform electric field. The α-particles enter the field with their velocity in the same direction as the field.
State and explain whether the magnitude of the acceleration of an α-particle due to the field decreases, increases or stays constant as the α-particle moves through the field.
e. A nucleus X is an isotope of a nucleus Y. The mass of nucleus X is greater than that of Y.
Both of the nuclei are in the same uniform electric field.
State and explain whether the magnitude of the electric force acting on nucleus X is greater than, less than or the same as that acting on nucleus Y.
a. The nucleus is charged (since it repels positively charged α-particles).
The majority of the mass of the atom is concentrated in the nucleus (only massive, dense regions can deflect α-particles by >90°).
b. A proton is not a fundamental particle because it is made up of three quarks (specifically: uud).
c. `Q = Ixxt = 6.9 xx 10^(-9) xx 60 = 4.14 xx 10^(-7) C`
Each α-particle has a charge of `2e = 2 xx 1.60 xx 10^(-19) " C"`
So number of α-particles: `Q / (2e) = (4.14 xx 10^(-7)) / (3.2 xx 10^(-19)) = 1.3 xx 10^12`
d. The electric field is uniform, so the force acting on each α-particle is constant.
Since mass is constant and F = ma, the acceleration is also constant.
e. Isotopes have same number of protons (same element) → same charge.
Electric force depends only on charge and field.
Hence, the electric force is the same on both nuclei.
Question 1
An isotope of boron decays to beryllium by β⁺ emission.
Which particle is emitted in addition to the β⁺ particle?
A. Antineutrino.
B. Electron.
C. Neutrino.
D. Neutron.
Question 2
Which statement about radioactive decay is correct?
A. Neutrinos are always emitted during α-decay.
B. The α-particles emitted from a radioactive sample have a continuous range of kinetic energies.
C. The β⁻ particles emitted from a radioactive sample have a continuous range of kinetic energies.
D. The proton number of a nucleus decreases by four when it undergoes α-decay.
Question 3
Nuclide X with proton number Z undergoes β⁺ decay to form nuclide Y.
The decay may be represented by the equation shown:
`X rightarrow Y + beta^++ W`"
What is the proton number of Y and which particle is represented by the symbol W?
| Proton number of Y | Particle represented by W | |
|---|---|---|
| A | Z − 1 | antineutrino |
| B | Z − 1 | neutrino |
| C | Z + 1 | antineutrino |
| D | Z + 1 | neutrino |
Question 4
The unstable nuclide 21884 decays through a sequence of emissions of α and β⁻ particles to form the stable nuclide 21083.
How many α and β⁻ particles are emitted during this decay process?
| α-particles | β⁻ particles | |
|---|---|---|
| A | 1 | 1 |
| B | 2 | 1 |
| C | 2 | 3 |
| D | 3 | 2 |
Question 5
What are isotopes?
A. Nuclei of different elements with the same number of neutrons.
B. Nuclei of different elements with the same number of nucleons.
C. Nuclei of the same element with different numbers of neutrons.
D. Nuclei of the same element with different numbers of protons.
Question 6
In an experiment on α-particle scattering, α-particles are directed at a thin gold foil. Most of the α-particles pass straight through the foil or are deflected by a small angle. A small number of α-particles are deflected by a large angle.
Which statement cannot be deduced from this experiment?
A. Atoms are mostly empty space.
B. Most of the mass of an atom is concentrated in the nucleus.
C. The nucleus of an atom contains protons.
D. The nucleus of an atom is small compared to the size of an atom.
Question 7
Which two particles have opposite charges?
A. Alpha-particle and helium nucleus
B. Antiproton and beta-plus particle
C. Beta-minus particle and electron
D. Positron and proton
Question 8
What is a conclusion from the alpha-particle scattering experiment?
A. Protons and electrons have equal but opposite charges.
B. Protons have a much larger mass than electrons.
C. The nucleus contains most of the mass of the atom.
D. The nucleus of an atom contains protons and neutrons.
Question 9
a. Compare an α-particle with a β⁺ particle in terms of their masses and charges.
b. Nucleus P undergoes α-decay to form nucleus Q. Nucleus Q then undergoes a further decay to form nucleus R. The proton and nucleon numbers of P and R are shown in figure.
 i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
i. On figure, draw a cross (×) to show the proton number and nucleon number of Q.
ii. State the names of the particles emitted as Q decays to form R.
c. Before the α-decay, P is travelling at a constant velocity. After the decay, Q has a velocity of 1.3 × 105 m.s−1 at an angle of 68∘ to the original path of P.
The α-particle has a velocity of 150 × 105 m.s−1 at an angle of θ to the original path of P, as shown in figure. i. Use the principle of conservation of momentum to determine θ.
i. Use the principle of conservation of momentum to determine θ.
ii.a Calculate the kinetic energy of the α‑particle.
Question 10
a. One of the results of the α-particle scattering experiment is that a very small minority of the α-particles are scattered through angles greater than 90°.
State what may be inferred about the structure of the atom from this result.
b. An α-particle is made up of other particles. One of these particles is a proton.
State and explain whether a proton is a fundamental particle.
c. A radioactive source produces a beam of α-particles in a vacuum. The average current produced by the beam is 6.9 × 10−9 A.
Calculate the average number of α-particles passing a fixed point in the beam in a time of 1.0 minute.
d. The α-particles in the vacuum in (c) enter a uniform electric field. The α-particles enter the field with their velocity in the same direction as the field.
State and explain whether the magnitude of the acceleration of an α-particle due to the field decreases, increases or stays constant as the α-particle moves through the field.
e. A nucleus X is an isotope of a nucleus Y. The mass of nucleus X is greater than that of Y.
Both of the nuclei are in the same uniform electric field.
State and explain whether the magnitude of the electric force acting on nucleus X is greater than, less than or the same as that acting on nucleus Y.