Question 1
Mass spectroscopy is an analytical technique that can be used to analyse elements and compounds.
Using mass spectroscopy, a sample of boron was found to contain two isotopes 10B and 11B with a relative abundance of 20% and 80% respectively.
Calculate the relative atomic mass of boron. Show your working.
Easy
Mark as Complete
Mark Scheme
Question 2
The mass spectrum of potassium is shown in
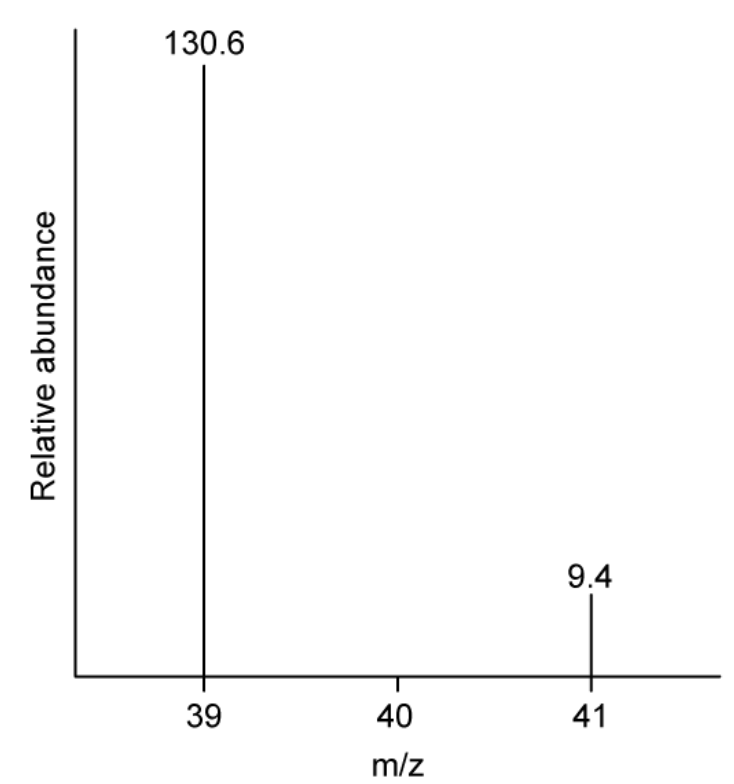
Calculate the relative atomic mass of potassium. Show your working
Easy
Mark as Complete
Mark Scheme
Question 3
a. The mass spectra of octane and butane were obtained and analysed.
Write the equation for the formation of the molecular ion of octane and predict its m/e value.
b. The mass spectrum of butane shows a molecular ion peak at m/e = 58.0.
Explain why there is also a smaller peak at m/e = 59.0 on the mass spectrum of butane
Medium
Mark as Complete
Mark Scheme
Question 4
State what the difference would be in the ratio of the peak heights of the M peak to the [M+2] peak in 1-bromobutane and 1-chlorobutane
Medium
Mark as Complete
Mark Scheme
Question 5
a. Compound X contains atoms of carbon, hydrogen and oxygen only.
The mass spectrum of X is recorded. Information about the two peaks with m/e greater than 100 is shown in the figure below
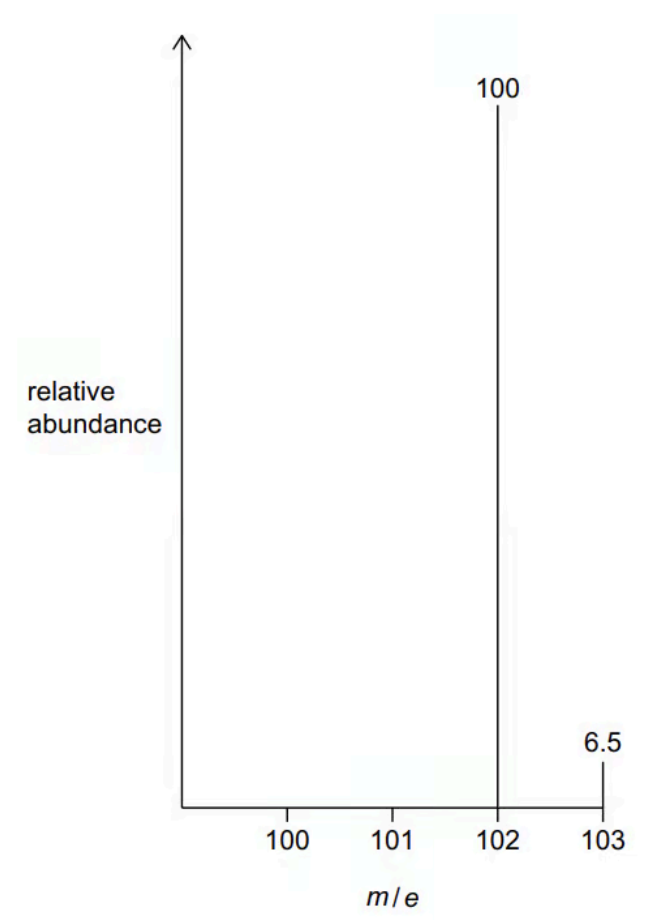
A molecule of X contains 6 carbon atoms.
Demonstrate that this is correct using information from the figure. Show your working
b. Suggest the molecular formula of X
c. Suggest the molecular formula of the fragment of X at m/e = 31
Hard
Mark as Complete
Mark Scheme
Question 6
a. The mass spectrum of magnesium is shown in
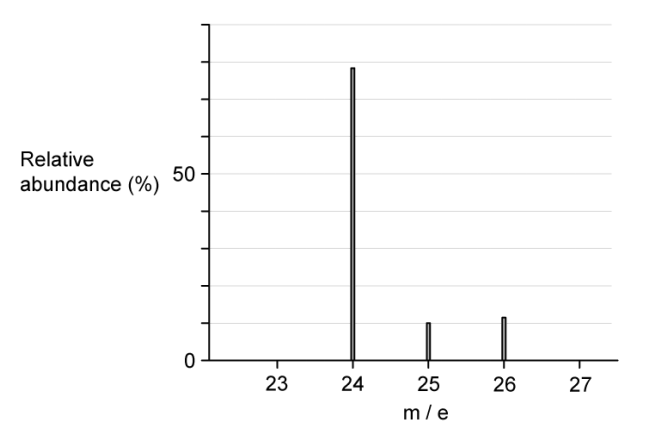
From the mass spectrum, complete the table with the relative abundances of the three isotopes
| isotope | relative abundance |
| 24Mg | |
| 25Mg | |
| 26Mg |
b. Use your values in (a) to calculate the relative atomic mass, Ar , of magnesium to two decimal places
Medium
Mark as Complete
Mark Scheme
Question 7
Infrared spectroscopy and mass spectrometry are used in the search for extra-terrestrial organic compounds
Compound X was analysed using both techniques.
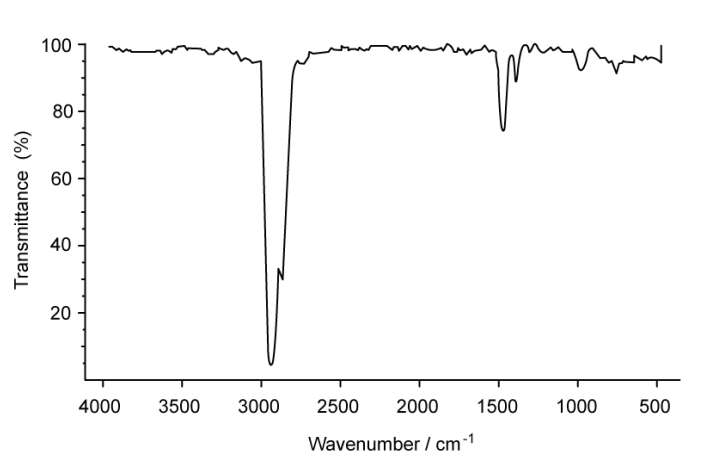
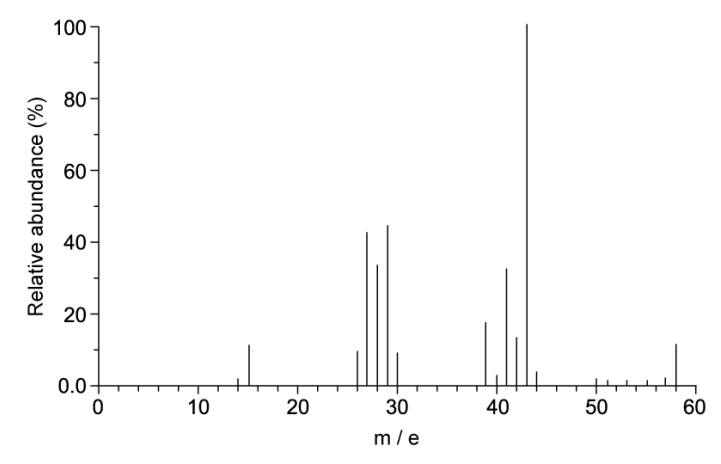
A. Suggest what evidence there is to support that compound X is a hydrocarbon
B. Explain how the mass spectrum supports that compound X has a molecular formula of C4H10
C. Draw the fully displayed formulae of two possible isomers for compound X
Hard
Mark as Complete
Mark Scheme
Question 8
Which of the following corresponds to the base peak in a mass spectrum?
A. The peak corresponding to the ion with lowest m/e
B. The peak corresponding to the molecular ion
C. The peak corresponding to any ion formed by the fragmentation of the molecule
D. The peak corresponding to the most abundant ion
Easy
Mark as Complete
Mark Scheme
Question 9
The diagram shows the structure of ricinoleic acid.

Which fragment would produce the tallest peak in the mass spectrum of ricinoleic acid at m/e = 55?
A. `CH_2CO""_2^+`
B. `CH═CHCHOH^+`
C. `C_4H""_7^+`
D. `COOH^+`
Medium
Mark as Complete
Mark Scheme
Question 10
In a mass spectrometer, the ions are separated.
Which of the following species will be deflected to the greatest extent?
A. 37Na+
B. 35Na+
C. 37Na
D. 35Na2+
Medium
Mark as Complete
Mark Scheme
Question 1
Mass spectroscopy is an analytical technique that can be used to analyse elements and compounds.
Using mass spectroscopy, a sample of boron was found to contain two isotopes 10B and 11B with a relative abundance of 20% and 80% respectively.
Calculate the relative atomic mass of boron. Show your working.
The relative atomic mass of boron = `((10 xx 20) + (11 xx 80)) / 100 = 10.8`
Question 2
The mass spectrum of potassium is shown in

Calculate the relative atomic mass of potassium. Show your working
The total abundance `= 130.6 + 9.4 = 140`
The relative atomic mass of potassium `= ((39 xx 130.6) + (41 xx 9.4)) / 140 = 39.1`
Question 3
a. The mass spectra of octane and butane were obtained and analysed.
Write the equation for the formation of the molecular ion of octane and predict its m/e value.
b. The mass spectrum of butane shows a molecular ion peak at m/e = 58.0.
Explain why there is also a smaller peak at m/e = 59.0 on the mass spectrum of butane
a. The equation for the formation of the molecular ion of octane
`C_8H_18 -> C_8H""_18^+ + e^-`
Thus, m/e value = `(8xx12)+(18xx1) = 114`
b. There is also a smaller peak at m/e = 59.0 on the mass spectrum of butane because there is 13C isotopes which will add a mass of 1 onto the molecular ion
Question 4
State what the difference would be in the ratio of the peak heights of the M peak to the [M+2] peak in 1-bromobutane and 1-chlorobutane
Because of the percentage of isotopes in nature.
As for chlorine, there are 2 isotopes in which 35Cl is 3 times more abundant than 37Cl , whereas bromine has 2 isotopes as well but their relative abundances are nearly equal.
That’s the reason why the ratio of the peak heights of the M peak to the [M+2] peak in 1-bromobutane would be 1:1 while that of in 1-chlorobutane would be 3:1
Question 5
a. Compound X contains atoms of carbon, hydrogen and oxygen only.
The mass spectrum of X is recorded. Information about the two peaks with m/e greater than 100 is shown in the figure below

A molecule of X contains 6 carbon atoms.
Demonstrate that this is correct using information from the figure. Show your working
b. Suggest the molecular formula of X
c. Suggest the molecular formula of the fragment of X at m/e = 31
a. `n = (100/1.1) xx (6.5/100) = 5.91 = 6.00`. Thus, there are 6 carbon atoms
b. The molecular formula of X
`102 - (6xx12) = 30`. Thus, there are 14 H and 1 O within this molecule, as such, C6H14O
c. We have some characteristic peaks including CH3+, CH2+, OH+ an so on. Therefore, the molecular formula of the fragment of X at m/e = 31, we can think of (CH2OH)+
Question 6
a. The mass spectrum of magnesium is shown in

From the mass spectrum, complete the table with the relative abundances of the three isotopes
| isotope | relative abundance |
| 24Mg | |
| 25Mg | |
| 26Mg |
b. Use your values in (a) to calculate the relative atomic mass, Ar , of magnesium to two decimal places
a.
| isotope | relative abundance |
| 24Mg | 79 |
| 25Mg | 10 |
| 26Mg | 11 |
b.
The relative atomic mass, Ar , of magnesium to two decimal places is
`(0.78 xx 24) + (0.10xx25) + (0.12xx26) = 24.34`
Question 7
Infrared spectroscopy and mass spectrometry are used in the search for extra-terrestrial organic compounds
Compound X was analysed using both techniques.


A. Suggest what evidence there is to support that compound X is a hydrocarbon
B. Explain how the mass spectrum supports that compound X has a molecular formula of C4H10
C. Draw the fully displayed formulae of two possible isomers for compound X
A. There are not any characteristic peaks in the IR spectrum except for around 2950 cm-1 which represents C-H bonds. Thus, there are no other functional groups
B. The mass spectrum supports that compound X has a molecular formula of C4H10 because there is a molecular ion peak at m/e 58 which belongs to C4H10
C. The fully displayed formulae of two possible isomers for compound X
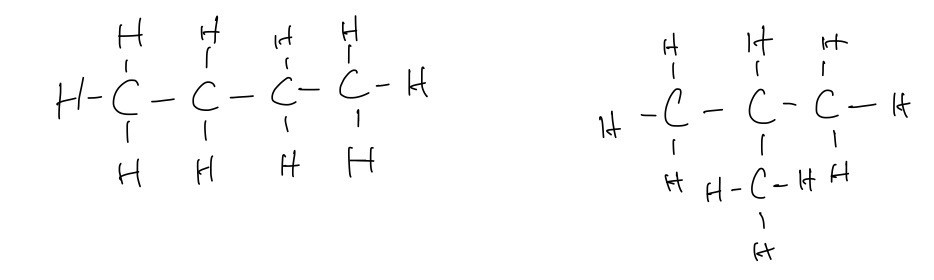
Question 8
Which of the following corresponds to the base peak in a mass spectrum?
A. The peak corresponding to the ion with lowest m/e
B. The peak corresponding to the molecular ion
C. The peak corresponding to any ion formed by the fragmentation of the molecule
D. The peak corresponding to the most abundant ion
The answer is D
A is incorrect because the base peak is not generated by the lowest m/e
B is incorrect because the peak representing the molecular ion is called the molecular ion peak, as such, the peak does not correspond to the molecular ion
C is incorrect because the base peak is not generated by any ion formed by the fragmentation of the molecule
Question 9
The diagram shows the structure of ricinoleic acid.

Which fragment would produce the tallest peak in the mass spectrum of ricinoleic acid at m/e = 55?
A. `CH_2CO""_2^+`
B. `CH═CHCHOH^+`
C. `C_4H""_7^+`
D. `COOH^+`
The answer is C
A is incorrect because this fragment has a peak at 58
B is incorrect because this fragment has a peak at 56
D is incorrect because this fragment has a peak at 45
Question 10
In a mass spectrometer, the ions are separated.
Which of the following species will be deflected to the greatest extent?
A. 37Na+
B. 35Na+
C. 37Na
D. 35Na2+
The answer is D
Species that will be deflected to the greatest extent are the ones with the smallest m/e value, as such, they will have the largest positive charge, leading to more attraction to the the negative pole in the MS
Question 1
Mass spectroscopy is an analytical technique that can be used to analyse elements and compounds.
Using mass spectroscopy, a sample of boron was found to contain two isotopes 10B and 11B with a relative abundance of 20% and 80% respectively.
Calculate the relative atomic mass of boron. Show your working.
Question 2
The mass spectrum of potassium is shown in

Calculate the relative atomic mass of potassium. Show your working
Question 3
a. The mass spectra of octane and butane were obtained and analysed.
Write the equation for the formation of the molecular ion of octane and predict its m/e value.
b. The mass spectrum of butane shows a molecular ion peak at m/e = 58.0.
Explain why there is also a smaller peak at m/e = 59.0 on the mass spectrum of butane
Question 4
State what the difference would be in the ratio of the peak heights of the M peak to the [M+2] peak in 1-bromobutane and 1-chlorobutane
Question 5
a. Compound X contains atoms of carbon, hydrogen and oxygen only.
The mass spectrum of X is recorded. Information about the two peaks with m/e greater than 100 is shown in the figure below

A molecule of X contains 6 carbon atoms.
Demonstrate that this is correct using information from the figure. Show your working
b. Suggest the molecular formula of X
c. Suggest the molecular formula of the fragment of X at m/e = 31
Question 6
a. The mass spectrum of magnesium is shown in

From the mass spectrum, complete the table with the relative abundances of the three isotopes
| isotope | relative abundance |
| 24Mg | |
| 25Mg | |
| 26Mg |
b. Use your values in (a) to calculate the relative atomic mass, Ar , of magnesium to two decimal places
Question 7
Infrared spectroscopy and mass spectrometry are used in the search for extra-terrestrial organic compounds
Compound X was analysed using both techniques.


A. Suggest what evidence there is to support that compound X is a hydrocarbon
B. Explain how the mass spectrum supports that compound X has a molecular formula of C4H10
C. Draw the fully displayed formulae of two possible isomers for compound X
Question 8
Which of the following corresponds to the base peak in a mass spectrum?
A. The peak corresponding to the ion with lowest m/e
B. The peak corresponding to the molecular ion
C. The peak corresponding to any ion formed by the fragmentation of the molecule
D. The peak corresponding to the most abundant ion
Question 9
The diagram shows the structure of ricinoleic acid.

Which fragment would produce the tallest peak in the mass spectrum of ricinoleic acid at m/e = 55?
A. `CH_2CO""_2^+`
B. `CH═CHCHOH^+`
C. `C_4H""_7^+`
D. `COOH^+`
Question 10
In a mass spectrometer, the ions are separated.
Which of the following species will be deflected to the greatest extent?
A. 37Na+
B. 35Na+
C. 37Na
D. 35Na2+