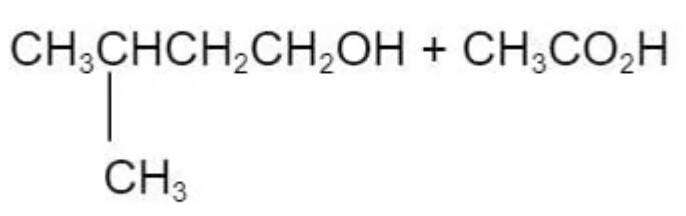Question 1
a. Complete the table to show how some common esters are formed.
| Carboxylic Acid | Alcohol | Ester |
| ethanoic acid | methyl ethanoate | |
| ethanol | ethyl propanoate | |
| butanoic acid | propan-1-ol |
b. All of the esters in part (a) are formed via a condensation reaction. State the conditions needed for this reaction.
c. Each of the esters in part (a) can be hydrolysed to reform the carboxylic acid and alcohol.
Give the reagents and conditions needed for this reaction
Easy
Mark as Complete
Mark Scheme
Question 2
Methyl ethanoate can also be hydrolysed under alkaline conditions using sodium hydroxide.
A. Draw the displayed formula of the salt and alcohol formed in this reaction.
B. How is the salt converted into carboxylic acid?
C. Apart from the conditions, and products made, give one other difference between acid and alkaline hydrolysis.
Easy
Mark as Complete
Mark Scheme
Question 3
a. An organic ester, B, has the empirical formula C2H4O. An experiment by a student in a college gave a value of 87.5 for Mr of B. What is the molecular formula of B?
b. Draw the structural formulae of four isomers of B that are esters
Medium
Mark as Complete
Mark Scheme
Question 4
The student hydrolysed his sample of C4H8O2 which has 4 isomers of ester by heating with aqueous mineral acid and then separating the alcohol, C, that was formed. He heated the alcohol C under reflux with acidified dichromate(VI) ions and collected the product D.
A sample of D gave an orange precipitate with 2,4-dinitrophenylhydrazine reagent.
A second sample of D gave no reaction with Tollens’ reagent.
A. What group does the reaction with 2,4-dinitrophenylhydrazine reagent show to be present in D?
B. What does the result of the test with Tollens’ reagent show about D?
C. What is the structural formula of the alcohol C?
D. Which of the ester isomers has the same structure as that of being mentioned?
Hard
Mark as Complete
Mark Scheme
Question 5
Esters are compounds which provide the flavour of many fruits and the perfumes of many flowers.
The ester `CH_3(CH_2)_2CO_2CH_3` contributes to the aroma of apples.
A. State the reagents and conditions needed for the hydrolysis of this ester.
B. Write the equation for the hydrolysis of this ester.
C. Apart from their use as perfumes and food flavourings, state one major commercial use of esters
Medium
Mark as Complete
Mark Scheme
Question 6
Leaf alcohol is a stereoisomer that can form when insects such as caterpillars eat green leaves.
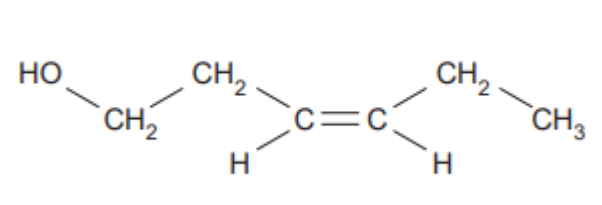
A. Draw the other stereo-isomer of leaf alcohol
B. Draw the structure for the ester formed when leaf alcohol reacts with ethanoic acid. Show all the bonds in the ester group
Medium
Mark as Complete
Mark Scheme
Question 7
Esters have important commercial uses, such as artificial flavourings in food.
They can be prepared in several ways, including through the reactions of alcohols with carboxylic acids, as well as the carboxylic acid derivatives, acid anhydrides and acyl chlorides.
Ester S, `CH_3CH_2COOCH_2CH(CH_3)_2` is used in rum flavouring.
Outline how you could obtain a sample of ester S, beginning with a named alcohol and carboxylic acid.
Include any essential reaction conditions and write an equation for the reaction.
You do not need to include details of the separation or purification methods involved
Medium
Mark as Complete
Mark Scheme
Question 8
A second ester, Ester T is responsible for a raspberry scent and has the molecular formula C5H10O2.
Ester T can be produced by the reaction of an acid with a branched primary alcohol.
Identify the acid and alcohol used to prepare ester T.
Draw and name ester T.
Medium
Mark as Complete
Mark Scheme
Question 9
The structure of ester E is shown in the diagram below.
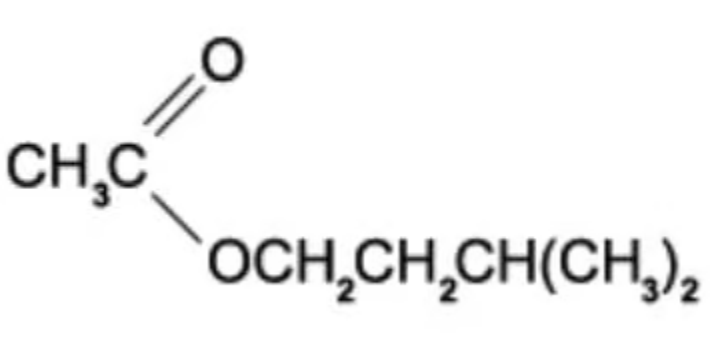
E is hydrolysed in the presence of hydrochloric acid.
Which are the products of this hydrolysis?
A. CH3CO2H and (CH3)2CHCH2CH2CHO
B. CH3CO2H and (CH3)2CHCH2CH2OH
C. CH3COCl and (CH3)2CHCH2CH2OH
D. CH3CHO and (CH3)2CHCH2CH2OH
Easy
Mark as Complete
Mark Scheme
Question 10
The ester in the diagram below has an odour of banana.
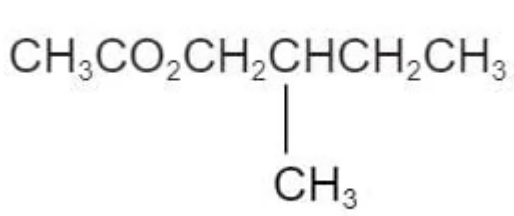
Which pair of reactants can be used to produce this ester under suitable conditions?
A. 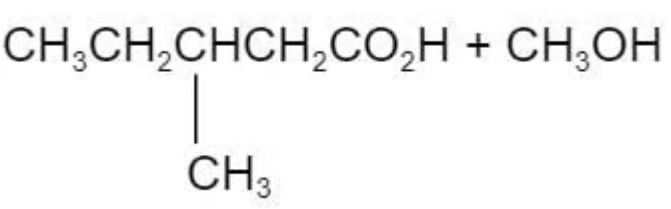
B. 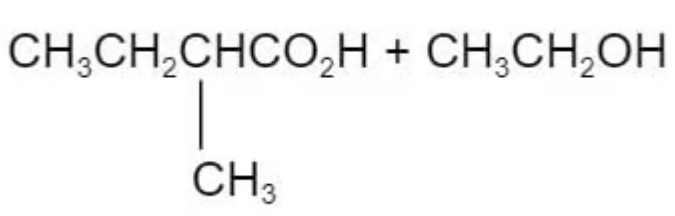
C. 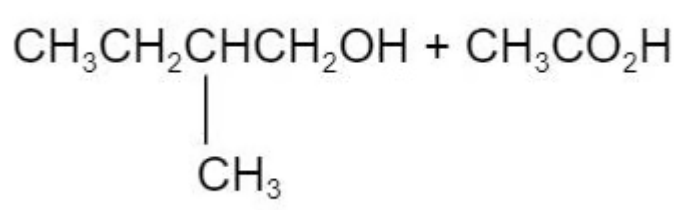
D. 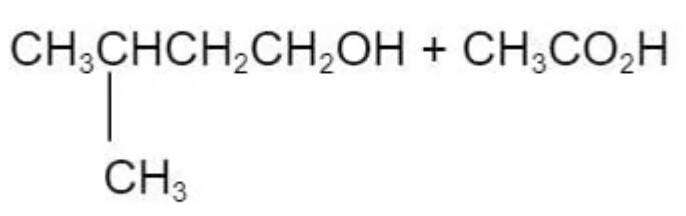
Easy
Mark as Complete
Mark Scheme
Question 1
a. Complete the table to show how some common esters are formed.
| Carboxylic Acid | Alcohol | Ester |
| ethanoic acid | methyl ethanoate | |
| ethanol | ethyl propanoate | |
| butanoic acid | propan-1-ol |
b. All of the esters in part (a) are formed via a condensation reaction. State the conditions needed for this reaction.
c. Each of the esters in part (a) can be hydrolysed to reform the carboxylic acid and alcohol.
Give the reagents and conditions needed for this reaction
a. The table to show how some common esters are formed
| Carboxylic Acid | Alcohol | Ester |
| ethanoic acid | methanol | methyl ethanoate |
| propanoic acid | ethanol | ethyl propanoate |
| butanoic acid | propan-1-ol | propyl butanoate |
b. The conditions needed for this reaction is concentrated H2SO4 and heat under refluxing
c. The reagents and conditions needed for this reaction include dilute acid or dilute alkali and heat under refluxing
Question 2
Methyl ethanoate can also be hydrolysed under alkaline conditions using sodium hydroxide.
A. Draw the displayed formula of the salt and alcohol formed in this reaction.
B. How is the salt converted into carboxylic acid?
C. Apart from the conditions, and products made, give one other difference between acid and alkaline hydrolysis.
A. The displayed formula of the salt and alcohol formed in this reaction

B. The salt is converted into carboxylic acid by adding dilute acid
C. One other difference between acid and alkaline hydrolysis is to establish an equilibrium in acidic hydrolysis
Question 3
a. An organic ester, B, has the empirical formula C2H4O. An experiment by a student in a college gave a value of 87.5 for Mr of B. What is the molecular formula of B?
b. Draw the structural formulae of four isomers of B that are esters
a. The Mr of the empirical formula = `2 xx 12.0 + 4 xx 1.0 + 1 xx 16.0 = 44`
The number of `n = 87.5 / 44 = 2`
Thus, the molecular formula of B is `C_4H_8O_2`
b. Structure 1: `HCO_2CH(CH_3)_2`
Structure 2: `HCO_2CH_2CH_2CH_3`
Structure 3: `CH_3CO_2CH_2CH_3`
Structure 4: `CH_3CH_2CO_2CH_3`
Question 4
The student hydrolysed his sample of C4H8O2 which has 4 isomers of ester by heating with aqueous mineral acid and then separating the alcohol, C, that was formed. He heated the alcohol C under reflux with acidified dichromate(VI) ions and collected the product D.
A sample of D gave an orange precipitate with 2,4-dinitrophenylhydrazine reagent.
A second sample of D gave no reaction with Tollens’ reagent.
A. What group does the reaction with 2,4-dinitrophenylhydrazine reagent show to be present in D?
B. What does the result of the test with Tollens’ reagent show about D?
C. What is the structural formula of the alcohol C?
D. Which of the ester isomers has the same structure as that of being mentioned?
A. The reaction with 2,4-dinitrophenylhydrazine reagent demonstrates the presence of carbonyl group
B. A second sample of D gave no reaction with Tollens’ reagent means that there is no aldehyde group formation but probably ketone present.
C. The structural formula of the alcohol C is (CH3)2CHOH
D. There are 4 isomers of C4H8O2 that we mention including `HCO_2CH(CH_3)_2, HCO_2CH_2CH_2CH_3, CH_3CO_2CH_2CH_3 and CH_3CH_2CO_2CH_3`. Thus, `HCO_2CH(CH_3)_2` is a suitable choice
Question 5
Esters are compounds which provide the flavour of many fruits and the perfumes of many flowers.
The ester `CH_3(CH_2)_2CO_2CH_3` contributes to the aroma of apples.
A. State the reagents and conditions needed for the hydrolysis of this ester.
B. Write the equation for the hydrolysis of this ester.
C. Apart from their use as perfumes and food flavourings, state one major commercial use of esters
A. The reagents and conditions needed for the hydrolysis of this ester include dilute acid or dilute alkali and heat under refluxing
B. The equation for the hydrolysis of this ester
`CH_3(CH_2)_2CO_2CH_3 + H_2O -> CH_3(CH_2)_2CO_2H + CH_3OH`
C. Major commercial use of esters could be mentioned to solvents or plastics
Question 6
Leaf alcohol is a stereoisomer that can form when insects such as caterpillars eat green leaves.

A. Draw the other stereo-isomer of leaf alcohol
B. Draw the structure for the ester formed when leaf alcohol reacts with ethanoic acid. Show all the bonds in the ester group
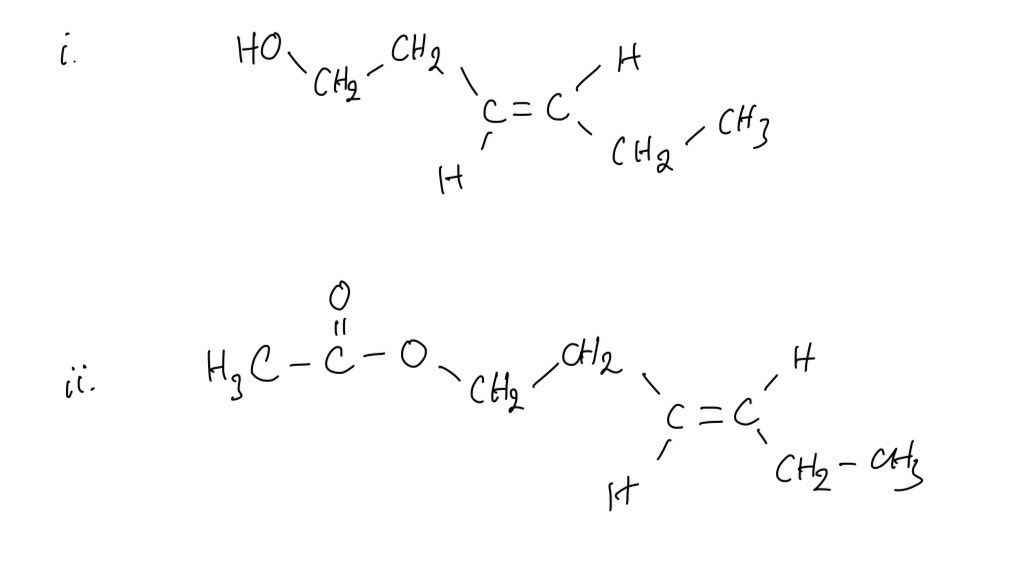
Question 7
Esters have important commercial uses, such as artificial flavourings in food.
They can be prepared in several ways, including through the reactions of alcohols with carboxylic acids, as well as the carboxylic acid derivatives, acid anhydrides and acyl chlorides.
Ester S, `CH_3CH_2COOCH_2CH(CH_3)_2` is used in rum flavouring.
Outline how you could obtain a sample of ester S, beginning with a named alcohol and carboxylic acid.
Include any essential reaction conditions and write an equation for the reaction.
You do not need to include details of the separation or purification methods involved
To obtain a sample of ester S, we need propanoic acid, 2-methylpropan-1-ol, heat under reflux and concentrated H2SO4
For an equation
`CH_3CH_2COOH + (CH_3)_2CHCH_2OH -> CH_3CH_2COOCH_2CH(CH_3)_2 + H_2O`
Question 8
A second ester, Ester T is responsible for a raspberry scent and has the molecular formula C5H10O2.
Ester T can be produced by the reaction of an acid with a branched primary alcohol.
Identify the acid and alcohol used to prepare ester T.
Draw and name ester T.
The acid and alcohol used to prepare ester T include formic acid and 2-methylpropan-1-ol
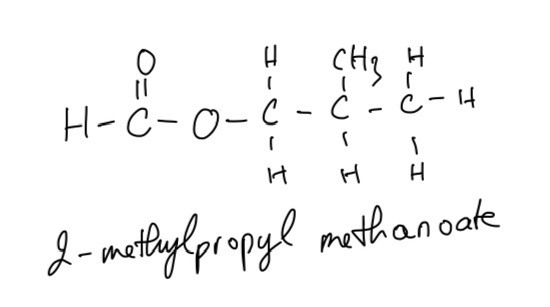
Question 9
The structure of ester E is shown in the diagram below.
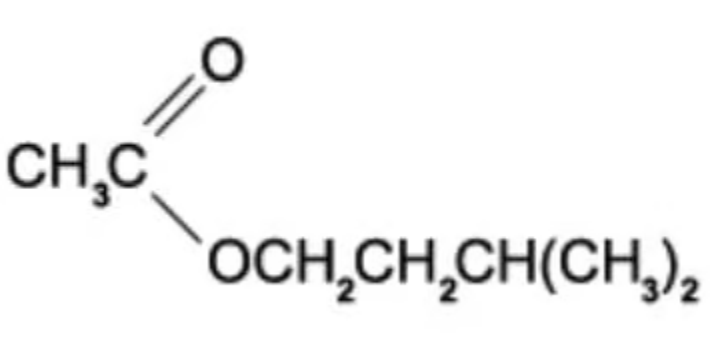
E is hydrolysed in the presence of hydrochloric acid.
Which are the products of this hydrolysis?
A. CH3CO2H and (CH3)2CHCH2CH2CHO
B. CH3CO2H and (CH3)2CHCH2CH2OH
C. CH3COCl and (CH3)2CHCH2CH2OH
D. CH3CHO and (CH3)2CHCH2CH2OH
The answer is B
A is incorrect because the ester hydrolysis is to create acid and alcohol
C is incorrect because the chlorine is a spectator ion
D is incorrect because hydrolysis of an ester is generating a carboxylic acid and alcohol.
Question 10
The ester in the diagram below has an odour of banana.

Which pair of reactants can be used to produce this ester under suitable conditions?
A. 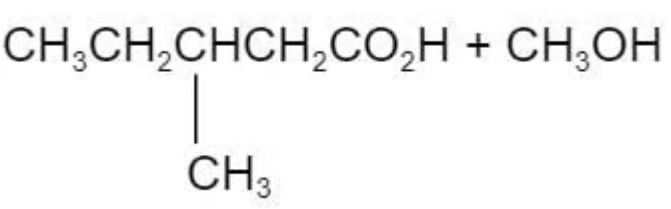
B. 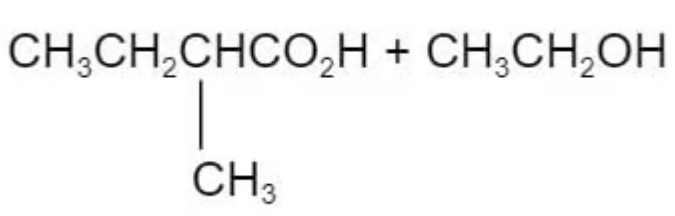
C. 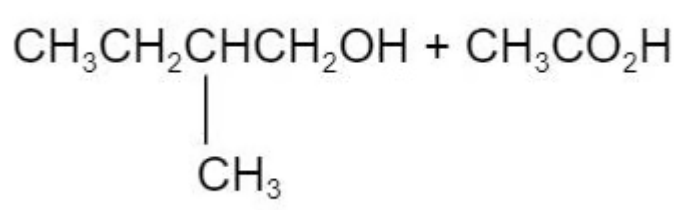
D. 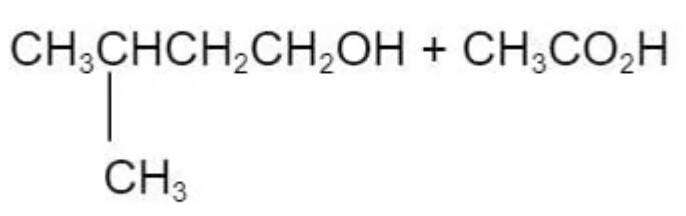
The answer is C
Ester is derived from carboxylic acid and alcohol.
A and B are incorrect because this is not the right combination of alcohol to the carboxylic acid
D is incorrect because the methyl group is attaching to the wrong carbon atom position
Question 1
a. Complete the table to show how some common esters are formed.
| Carboxylic Acid | Alcohol | Ester |
| ethanoic acid | methyl ethanoate | |
| ethanol | ethyl propanoate | |
| butanoic acid | propan-1-ol |
b. All of the esters in part (a) are formed via a condensation reaction. State the conditions needed for this reaction.
c. Each of the esters in part (a) can be hydrolysed to reform the carboxylic acid and alcohol.
Give the reagents and conditions needed for this reaction
Question 2
Methyl ethanoate can also be hydrolysed under alkaline conditions using sodium hydroxide.
A. Draw the displayed formula of the salt and alcohol formed in this reaction.
B. How is the salt converted into carboxylic acid?
C. Apart from the conditions, and products made, give one other difference between acid and alkaline hydrolysis.
Question 3
a. An organic ester, B, has the empirical formula C2H4O. An experiment by a student in a college gave a value of 87.5 for Mr of B. What is the molecular formula of B?
b. Draw the structural formulae of four isomers of B that are esters
Question 4
The student hydrolysed his sample of C4H8O2 which has 4 isomers of ester by heating with aqueous mineral acid and then separating the alcohol, C, that was formed. He heated the alcohol C under reflux with acidified dichromate(VI) ions and collected the product D.
A sample of D gave an orange precipitate with 2,4-dinitrophenylhydrazine reagent.
A second sample of D gave no reaction with Tollens’ reagent.
A. What group does the reaction with 2,4-dinitrophenylhydrazine reagent show to be present in D?
B. What does the result of the test with Tollens’ reagent show about D?
C. What is the structural formula of the alcohol C?
D. Which of the ester isomers has the same structure as that of being mentioned?
Question 5
Esters are compounds which provide the flavour of many fruits and the perfumes of many flowers.
The ester `CH_3(CH_2)_2CO_2CH_3` contributes to the aroma of apples.
A. State the reagents and conditions needed for the hydrolysis of this ester.
B. Write the equation for the hydrolysis of this ester.
C. Apart from their use as perfumes and food flavourings, state one major commercial use of esters
Question 6
Leaf alcohol is a stereoisomer that can form when insects such as caterpillars eat green leaves.

A. Draw the other stereo-isomer of leaf alcohol
B. Draw the structure for the ester formed when leaf alcohol reacts with ethanoic acid. Show all the bonds in the ester group
Question 7
Esters have important commercial uses, such as artificial flavourings in food.
They can be prepared in several ways, including through the reactions of alcohols with carboxylic acids, as well as the carboxylic acid derivatives, acid anhydrides and acyl chlorides.
Ester S, `CH_3CH_2COOCH_2CH(CH_3)_2` is used in rum flavouring.
Outline how you could obtain a sample of ester S, beginning with a named alcohol and carboxylic acid.
Include any essential reaction conditions and write an equation for the reaction.
You do not need to include details of the separation or purification methods involved
Question 8
A second ester, Ester T is responsible for a raspberry scent and has the molecular formula C5H10O2.
Ester T can be produced by the reaction of an acid with a branched primary alcohol.
Identify the acid and alcohol used to prepare ester T.
Draw and name ester T.
Question 9
The structure of ester E is shown in the diagram below.
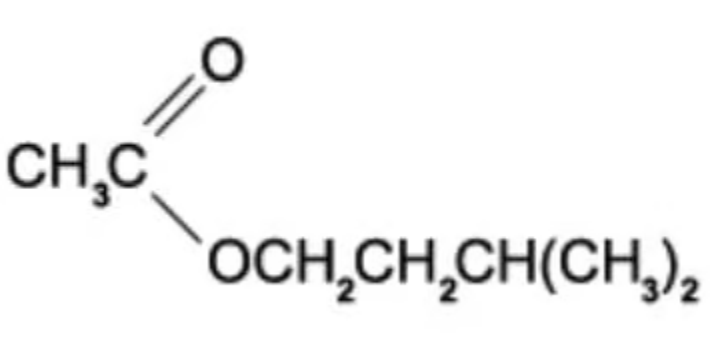
E is hydrolysed in the presence of hydrochloric acid.
Which are the products of this hydrolysis?
A. CH3CO2H and (CH3)2CHCH2CH2CHO
B. CH3CO2H and (CH3)2CHCH2CH2OH
C. CH3COCl and (CH3)2CHCH2CH2OH
D. CH3CHO and (CH3)2CHCH2CH2OH
Question 10
The ester in the diagram below has an odour of banana.

Which pair of reactants can be used to produce this ester under suitable conditions?
A. 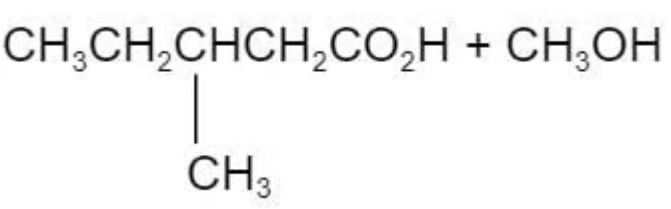
B. 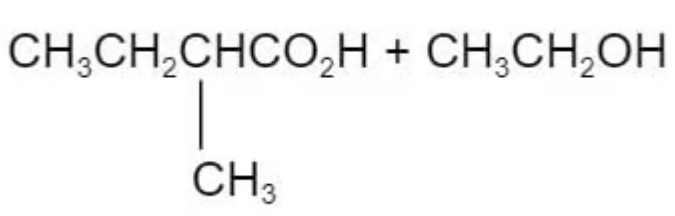
C. 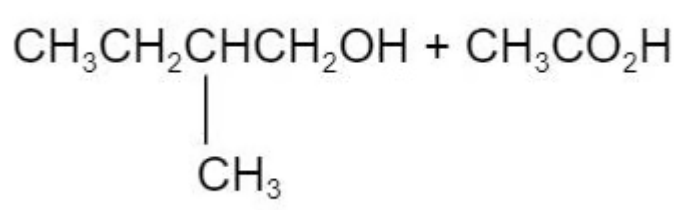
D.