Question 1
3-ethylpentane, 2-methylpentane, and 2,3-dimethylbutane are alkanes.
For each one give:
i. its molecular formula
ii. its structural formula
iii. its displayed formula
iv. its skeletal formula
Easy
Mark as Complete
Mark Scheme
Question 2
A. In 2-methylpentane, 3-ethylpentane and 2,3-dimethylbutane, two of the alkanes in this question are isomers of each other. Identify which two and identify the type of isomerism they show.
B. Define isomers
C. Name the alkane whose structural formula is `CH_3CH(CH_3)CH_2CH(CH_3)_2`
Easy
Mark as Complete
Mark Scheme
Question 3
A. Alkanes are saturated hydrocarbons. Explain the words saturated and hydrocarbons.
B. Alkanes are generally unreactive. Explain why this is so.
Easy
Mark as Complete
Mark Scheme
Question 4
Use the passage below and your own knowledge to answer the questions that follow. Methane reacts with bromine to give bromomethane and hydrogen bromide. The mechanism for the reaction is called free-radical substitution and involves homolytic fission of chemical bonds. The reaction proceeds via initiation, propagation and termination steps.
a. By what mechanism does bromine react with methane?
b. Write a balanced symbol equation for this reaction.
c. Bonds break in this reaction. What type of bond breaking is involved?
d. What essential conditions are required for this reaction? Why?
Medium
Mark as Complete
Mark Scheme
Question 5
Write down an equation that methane reacts with chlorine for:
A. an initiation step
B. a propagation step
C. a termination step
Medium
Mark as Complete
Mark Scheme
Question 6
1.50 g of ethane reacts with chlorine. 1.29 g of chloroethane is formed.
(Ar values: H = 1.0, C = 12.0, Cl = 35.5)
Calculate:
a. the number of moles of ethane that were used
b. the number of moles of chloroethane that were formed
c. the percentage yield
d. the number of grams of chloroethane that would have formed if the percentage yield had been 60.0 %.
Medium
Mark as Complete
Mark Scheme
Question 7
One of the reactions taking place in a catalytic converter in a car exhaust system is between nitrogen oxide and octane (unburned petrol). The products of this reaction are non-toxic.
Which is the correct equation for the reaction?
A. `C_8H_16 + 16NO → 8CO + 8N_2 + 8H_2O`
B. `C_8H_16 + 24NO → 8CO_2 + 12N_2 + 8H_2O`
C. `C_8H_18 + 17NO → 8CO + 8 1/2 N_2 + 9H_2O`
D. `C_8H_18 + 25NO → 8CO_2 + 12 1/2N_2 + 9H_2O`
Easy
Mark as Complete
Mark Scheme
Question 8
The hydrocarbon C17H36 can be cracked. Which compound is the least likely to be produced in this reaction?
A. C3H8
B. C4H8
C. C8H16
D. C16H34
Medium
Mark as Complete
Mark Scheme
Question 9
In the presence of ultraviolet light, ethane and chlorine react to give a mixture of products.
Which compound could be present in the mixture of products?
A. CH3Cl
B. CH3CH2CH2Cl
C. CH3CH2CH2CH3
D. CH3CH2CH2CH2CH3
Medium
Mark as Complete
Mark Scheme
Question 10
The cracking of a single hydrocarbon molecule, CnH2n+2 , produces two hydrocarbon molecules only. Each hydrocarbon product contains the same number of carbon atoms in one molecule. Each hydrocarbon product has non-cyclic structural isomers.
What is the value of n?
A. 9
B. 8
C. 6
D. 4
Hard
Mark as Complete
Mark Scheme
Question 1
3-ethylpentane, 2-methylpentane, and 2,3-dimethylbutane are alkanes.
For each one give:
i. its molecular formula
ii. its structural formula
iii. its displayed formula
iv. its skeletal formula
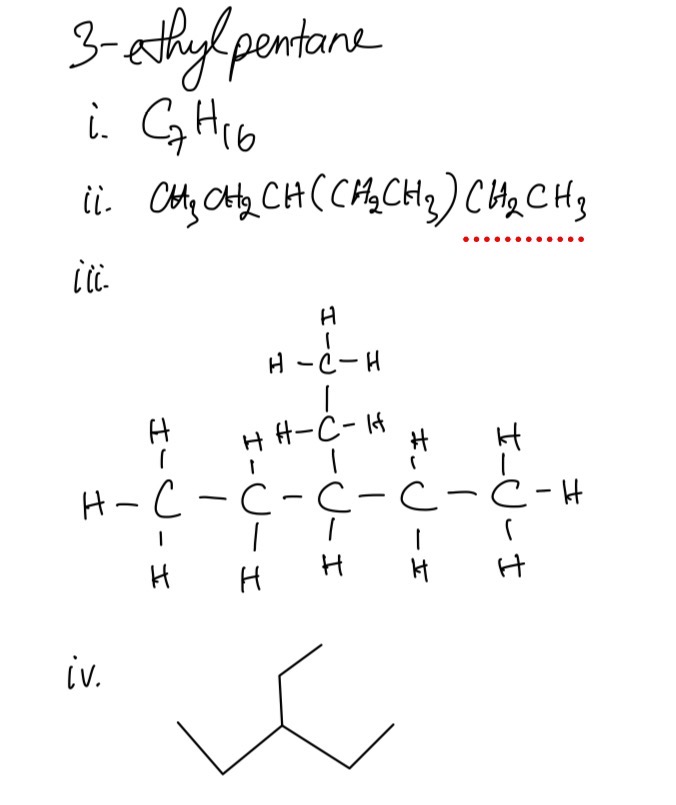
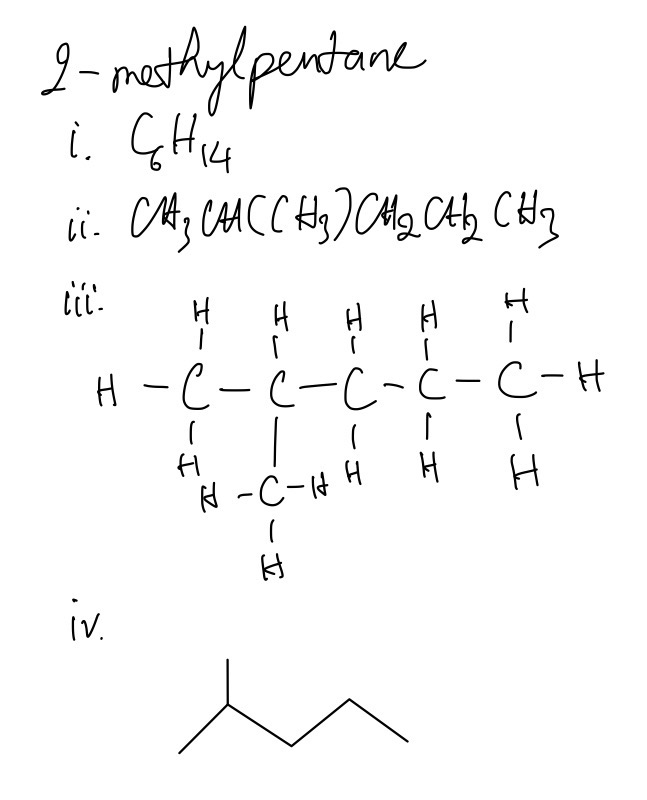
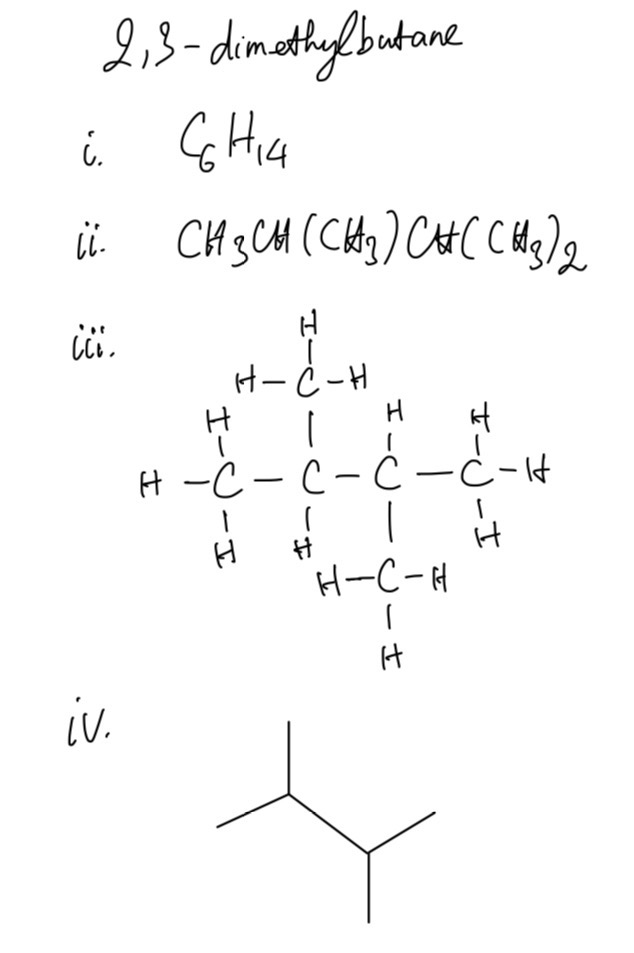
Question 2
A. In 2-methylpentane, 3-ethylpentane and 2,3-dimethylbutane, two of the alkanes in this question are isomers of each other. Identify which two and identify the type of isomerism they show.
B. Define isomers
C. Name the alkane whose structural formula is `CH_3CH(CH_3)CH_2CH(CH_3)_2`
A. Two of the alkanes which are isomers of each other are 2-methylpentane and 2,3-dimethylbutane. They belong to structural isomerism
B. Isomers are compounds with the same molecular formula but different structural formulae
C. That’s 2,4-dimethylpentane
Question 3
A. Alkanes are saturated hydrocarbons. Explain the words saturated and hydrocarbons.
B. Alkanes are generally unreactive. Explain why this is so.
A. The word saturated means only single bonds and the word hydrocarbons means that a compound contains C and H only
B. Alkanes are generally unreactive because they lack the polarity of the C-H bond.
Question 4
Use the passage below and your own knowledge to answer the questions that follow. Methane reacts with bromine to give bromomethane and hydrogen bromide. The mechanism for the reaction is called free-radical substitution and involves homolytic fission of chemical bonds. The reaction proceeds via initiation, propagation and termination steps.
a. By what mechanism does bromine react with methane?
b. Write a balanced symbol equation for this reaction.
c. Bonds break in this reaction. What type of bond breaking is involved?
d. What essential conditions are required for this reaction? Why?
a. Mechanism that bromine reacts with methane is free-radical substitution
b. A balanced symbol equation for this reaction
`CH_4 + Br_2 -> CH_3Br + HBr`
c. The type of bond breaking is homolytic fission
d. For free-radical substitution, we need UV/light to break down the Br-Br bond
Question 5
Write down an equation that methane reacts with chlorine for:
A. an initiation step
B. a propagation step
C. a termination step
A. For an initiation step
`Br_2 -> 2Br*`
B. For a propagation step
`Br* + CH_4 -> CH_3* +HBr`
C. For a termination step
`CH3 * + Br * -> CH_3Br`
Question 6
1.50 g of ethane reacts with chlorine. 1.29 g of chloroethane is formed.
(Ar values: H = 1.0, C = 12.0, Cl = 35.5)
Calculate:
a. the number of moles of ethane that were used
b. the number of moles of chloroethane that were formed
c. the percentage yield
d. the number of grams of chloroethane that would have formed if the percentage yield had been 60.0 %.
`C_2H_6 + Cl_2 -> C_2H_5Cl + HCl`
a. The number of moles of ethane = `1.50 / 30.0 = 0.0500` moles
b. The number of moles of chloroethane = `1.29 / 64.5 = 0.0200` moles
c. The percentage yield = `0.0200 / 0.0500 xx 100% = 40.0 %`
d. The number of moles of chloroethane would have to get percentage yield of `60.0 % = 0.6 xx 0.05 = 0.03` moles
The number of grams of chloroethane = `0.03 xx 64.5 = 1.935` g
Question 7
One of the reactions taking place in a catalytic converter in a car exhaust system is between nitrogen oxide and octane (unburned petrol). The products of this reaction are non-toxic.
Which is the correct equation for the reaction?
A. `C_8H_16 + 16NO → 8CO + 8N_2 + 8H_2O`
B. `C_8H_16 + 24NO → 8CO_2 + 12N_2 + 8H_2O`
C. `C_8H_18 + 17NO → 8CO + 8 1/2 N_2 + 9H_2O`
D. `C_8H_18 + 25NO → 8CO_2 + 12 1/2N_2 + 9H_2O`
The answer is D
A and B are incorrect because octane is an alkane with formula C8H18
C is incorrect because CO is a toxic gas
Question 8
The hydrocarbon C17H36 can be cracked. Which compound is the least likely to be produced in this reaction?
A. C3H8
B. C4H8
C. C8H16
D. C16H34
The answer is D
Cracking is a reaction in which the large, less useful hydrocarbon molecules are broken down into smaller, more useful molecules.
Thus, for C17H36, the cracking process is likely to produce alkanes and alkenes of less than 10 carbons.
Question 9
In the presence of ultraviolet light, ethane and chlorine react to give a mixture of products.
Which compound could be present in the mixture of products?
A. CH3Cl
B. CH3CH2CH2Cl
C. CH3CH2CH2CH3
D. CH3CH2CH2CH2CH3
The answer is C
Inititation step: `Cl_2 -> 2Cl*`
Propagation step: `C_2H_6 + Cl* -> C_2H_5* + HCl`
Termination step: `C_2H_5* + C_2H_5* -> C_4H_10` which represents `CH_3CH_2CH_2CH_3`
A is incorrect because methane will involve reaction instead
B is incorrect because propane will involve reaction instead
D is incorrect because both ethane and propane will involve reaction
Question 10
The cracking of a single hydrocarbon molecule, CnH2n+2 , produces two hydrocarbon molecules only. Each hydrocarbon product contains the same number of carbon atoms in one molecule. Each hydrocarbon product has non-cyclic structural isomers.
What is the value of n?
A. 9
B. 8
C. 6
D. 4
The answer is B
C8H18 in cracking process will have 4 carbon atoms (butene) and 4 atoms (butane)
Each hydrocarbon product has non-cyclic structural isomers which butene does include but-1-ene, cis-but-2-ene, trans-but-2-ene, isobutene.
A is incorrect because n must be even due to each hydrocarbon product that contains the same number of carbon atoms in one molecule
C is incorrect because the cracked molecules will have 3 carbon atoms which will have 2 isomers for alkene molecules, one of which is cyclopropane and another is a cyclic isomer
D is incorrect because the cracked molecules will have 2 atoms which has no isomers for alkeme molecules
Question 1
3-ethylpentane, 2-methylpentane, and 2,3-dimethylbutane are alkanes.
For each one give:
i. its molecular formula
ii. its structural formula
iii. its displayed formula
iv. its skeletal formula
Question 2
A. In 2-methylpentane, 3-ethylpentane and 2,3-dimethylbutane, two of the alkanes in this question are isomers of each other. Identify which two and identify the type of isomerism they show.
B. Define isomers
C. Name the alkane whose structural formula is `CH_3CH(CH_3)CH_2CH(CH_3)_2`
Question 3
A. Alkanes are saturated hydrocarbons. Explain the words saturated and hydrocarbons.
B. Alkanes are generally unreactive. Explain why this is so.
Question 4
Use the passage below and your own knowledge to answer the questions that follow. Methane reacts with bromine to give bromomethane and hydrogen bromide. The mechanism for the reaction is called free-radical substitution and involves homolytic fission of chemical bonds. The reaction proceeds via initiation, propagation and termination steps.
a. By what mechanism does bromine react with methane?
b. Write a balanced symbol equation for this reaction.
c. Bonds break in this reaction. What type of bond breaking is involved?
d. What essential conditions are required for this reaction? Why?
Question 5
Write down an equation that methane reacts with chlorine for:
A. an initiation step
B. a propagation step
C. a termination step
Question 6
1.50 g of ethane reacts with chlorine. 1.29 g of chloroethane is formed.
(Ar values: H = 1.0, C = 12.0, Cl = 35.5)
Calculate:
a. the number of moles of ethane that were used
b. the number of moles of chloroethane that were formed
c. the percentage yield
d. the number of grams of chloroethane that would have formed if the percentage yield had been 60.0 %.
Question 7
One of the reactions taking place in a catalytic converter in a car exhaust system is between nitrogen oxide and octane (unburned petrol). The products of this reaction are non-toxic.
Which is the correct equation for the reaction?
A. `C_8H_16 + 16NO → 8CO + 8N_2 + 8H_2O`
B. `C_8H_16 + 24NO → 8CO_2 + 12N_2 + 8H_2O`
C. `C_8H_18 + 17NO → 8CO + 8 1/2 N_2 + 9H_2O`
D. `C_8H_18 + 25NO → 8CO_2 + 12 1/2N_2 + 9H_2O`
Question 8
The hydrocarbon C17H36 can be cracked. Which compound is the least likely to be produced in this reaction?
A. C3H8
B. C4H8
C. C8H16
D. C16H34
Question 9
In the presence of ultraviolet light, ethane and chlorine react to give a mixture of products.
Which compound could be present in the mixture of products?
A. CH3Cl
B. CH3CH2CH2Cl
C. CH3CH2CH2CH3
D. CH3CH2CH2CH2CH3
Question 10
The cracking of a single hydrocarbon molecule, CnH2n+2 , produces two hydrocarbon molecules only. Each hydrocarbon product contains the same number of carbon atoms in one molecule. Each hydrocarbon product has non-cyclic structural isomers.
What is the value of n?
A. 9
B. 8
C. 6
D. 4