Question 1
A carbon compound P has the percentage composition 85.7 % carbon and 14.3 % hydrogen. Its relative molecular mass was found to be 56.
a.
i. Calculate its empirical formula.
ii. Calculate its molecular formula.
b. Write down the names and displayed formulae of all the non-cyclic isomers of compound P which have the following characteristics:
i. straight chain
ii. branched chain.
Medium
Mark as Complete
Mark Scheme
Question 2
He reacted ethane with chlorine in the presence of UV light by the following reaction:
`C _2H_6(g) + 2Cl_2(g) → C_2H_4Cl_2(l) + 2HCl(g)`
After doing this he found that 600 g of ethane gave 148.5 g of C2H4Cl2.
A. How many moles of ethane are there in 600 g?
B. How many moles of 1,2-dichloroethane would have been formed if the yield had been 100 %?
C. How many moles of 1,2-dichloroethane are there in 148.5 g?
D. Calculate the percentage yield of 1,2-dichloroethane.
Medium
Mark as Complete
Mark Scheme
Question 3
He reacted ethene with chlorine in the dark by the following reaction:
`C_2H_4(g) + Cl_2(g) -> C_2H_4Cl_2(l)`
In this reaction 140 g of ethene gave 396 g of C2H4Cl2.
A. Calculate the percentage yield for this reaction. Show your working.
B. There are isomers of the compound C2H4Cl2. Draw the displayed formulae of the isomers and name them.
C. Choose from redox, substitution, elimination, addition and hydrolysis to give the type of reaction for this reaction
Medium
Mark as Complete
Mark Scheme
Question 4
X can be manufactured by the oxidation of propene.
`CH_3CHCH_2 + O_2 → CH_2CHCHO + H_2O`
Name X and state the functional groups that are present.
Medium
Mark as Complete
Mark Scheme
Question 5
a. Crotonic acid, C4H6O2, is used in the manufacture of paints and adhesives.
State the empirical formula of crotonic acid.
b. The systematic name of crotonic acid is but-2-enoic acid.
Draw two displayed formulae for possible isomers of crotonic acid that retain the carboxylic acid functional group.
Medium
Mark as Complete
Mark Scheme
Question 6
Z is a saturated hydrocarbon that is an isomer of pentene. The molecular formula of Z matches the general formula of the homologous series of alkenes.
Draw the skeletal formula of Z.
Medium
Mark as Complete
Mark Scheme
Question 7
What is the identity of the functional group that is ringed in the molecule shown below?
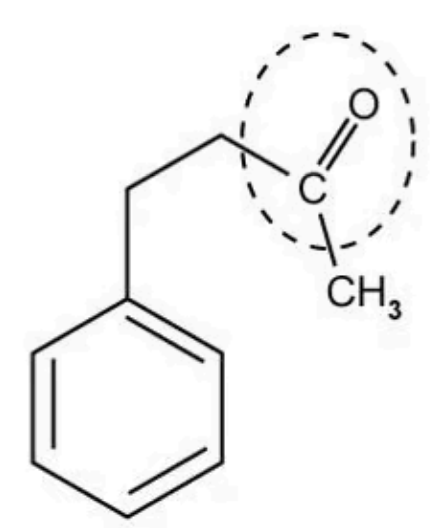
A. Aldehyde
B. Ketone
C. Carboxylic acid
D. Alkene
Medium
Mark as Complete
Mark Scheme
Question 8
What is the IUPAC name of the following molecule?
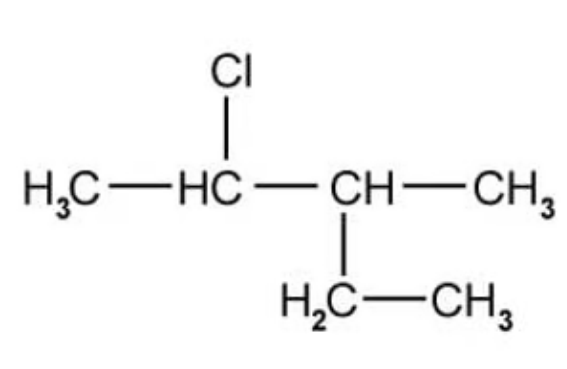
A. 2-chloro-3-methylpentane
B. 3-methyl-4-chloropentane
C. 2-ethyl-3-chlorobutane
D. 2-chloro-3-ethylbutane
Medium
Mark as Complete
Mark Scheme
Question 9
Which of the following molecules are hydrocarbons?
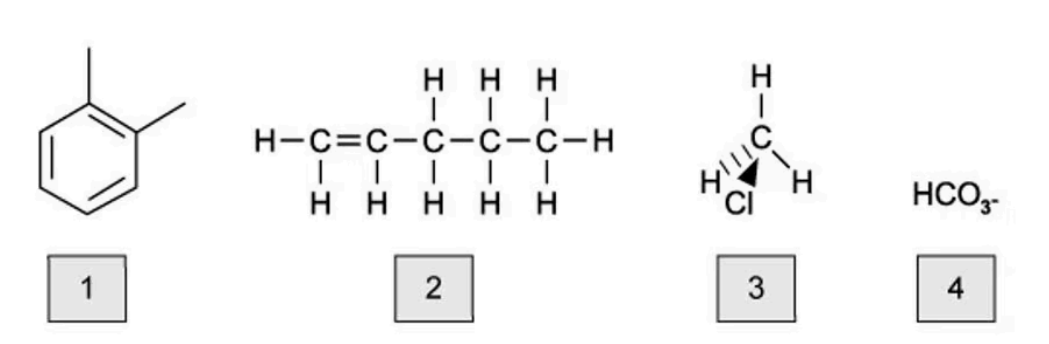
A. 1 and 2
B. 1, 2, 3 and 4
C. 1, 2 and 3
D. 4 only
Easy
Mark as Complete
Mark Scheme
Question 10
What is the correct formula for 1-fluro-3-methyl-2-butene?
| A. | Structural | `CH_2FCH_2 C(CH _3)CH_3` |
| B. | Empirical | C5H9F |
| C. | Displayed | 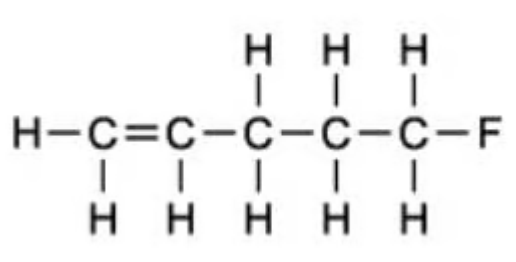 |
| D. | Skeletal | 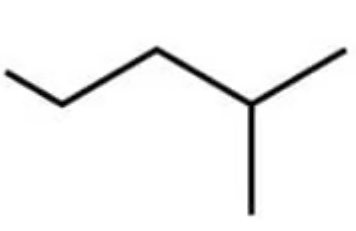 |
Medium
Mark as Complete
Mark Scheme
Question 1
A carbon compound P has the percentage composition 85.7 % carbon and 14.3 % hydrogen. Its relative molecular mass was found to be 56.
a.
i. Calculate its empirical formula.
ii. Calculate its molecular formula.
b. Write down the names and displayed formulae of all the non-cyclic isomers of compound P which have the following characteristics:
i. straight chain
ii. branched chain.
a.
i. we have the relative mass of carbon, Ar = 12.0, and the relative mass of hydrogen, Ar = 1.0
We assume that there are 100g of compound P.
thus, there are 85.7 g of C per 100g of compound and 14.3 g of H per 100g of compound.
The number of moles of C = `85.7 / 12.0` = 7.14 moles
The number of moles of H = `14.3 / 1.0` = 14.3 moles
The ratio between number of these atoms
C = `7.14 / 7.14` = 1
H = `14.3 / 7.14` = 2
Thus, the empirical formula is CH2
ii. The relative molecular mass of this empirical formula is `12.0 + 2 xx 1.0 = 14.0`
We know that its relative molecular mass was found to be 56. Therefore, the number of units of the empirical formula is `56 / 14.0 = 4`
Thus, the molecular formula is C4H8
b.
i.
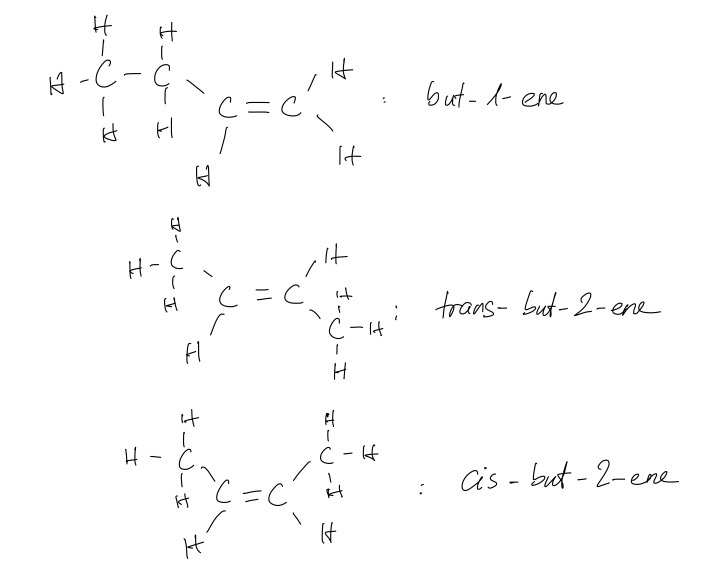
ii.
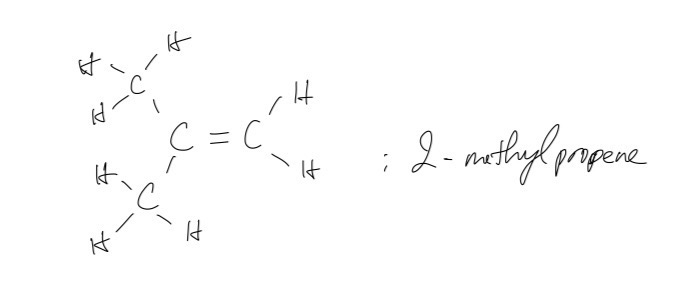
Question 2
He reacted ethane with chlorine in the presence of UV light by the following reaction:
`C _2H_6(g) + 2Cl_2(g) → C_2H_4Cl_2(l) + 2HCl(g)`
After doing this he found that 600 g of ethane gave 148.5 g of C2H4Cl2.
A. How many moles of ethane are there in 600 g?
B. How many moles of 1,2-dichloroethane would have been formed if the yield had been 100 %?
C. How many moles of 1,2-dichloroethane are there in 148.5 g?
D. Calculate the percentage yield of 1,2-dichloroethane.
A. The number of moles of ethane = `600 / 30`= 20 moles
B. The ratio of moles between ethane and 1,2-dichloroethane is 1:1, as such, to obtain 100 % of yield, the moles of 1,2-dichloroethane = the moles of ethane = 20 moles
C. The number of moles of 1,2-dichloroethane in 148.5 g = `148.5 / 99` = 1.5 moles
D The percentage yield of 1,2-dichloroethane = `1.5 / 20 xx 100%` = 7.5 %
Question 3
He reacted ethene with chlorine in the dark by the following reaction:
`C_2H_4(g) + Cl_2(g) -> C_2H_4Cl_2(l)`
In this reaction 140 g of ethene gave 396 g of C2H4Cl2.
A. Calculate the percentage yield for this reaction. Show your working.
B. There are isomers of the compound C2H4Cl2. Draw the displayed formulae of the isomers and name them.
C. Choose from redox, substitution, elimination, addition and hydrolysis to give the type of reaction for this reaction
A.
The number of moles of ethene = `140 / 28` = 5 moles
The number of moles of C2H4Cl2 = `396 / 99` = 4 moles
The percentage yield of this reaction = `4/5 xx 100%` = 80 %
B.
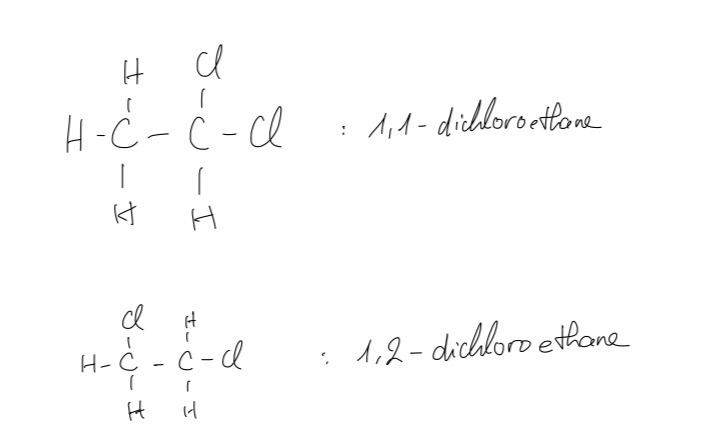
C. The type of reactions for this reaction is addition which involves the formation of a single product from two reactant molecules.
Question 4
X can be manufactured by the oxidation of propene.
`CH_3CHCH_2 + O_2 → CH_2CHCHO + H_2O`
Name X and state the functional groups that are present.
The name of X is propenal
The functional groups that are present in the molecule are alkene and aldehyde
Question 5
a. Crotonic acid, C4H6O2, is used in the manufacture of paints and adhesives.
State the empirical formula of crotonic acid.
b. The systematic name of crotonic acid is but-2-enoic acid.
Draw two displayed formulae for possible isomers of crotonic acid that retain the carboxylic acid functional group.
a. The empirical formula of crotonic acid is C2H3O
b. The displayed formulae for possible isomers of crotonic acid that retain the carboxylic acid functional group
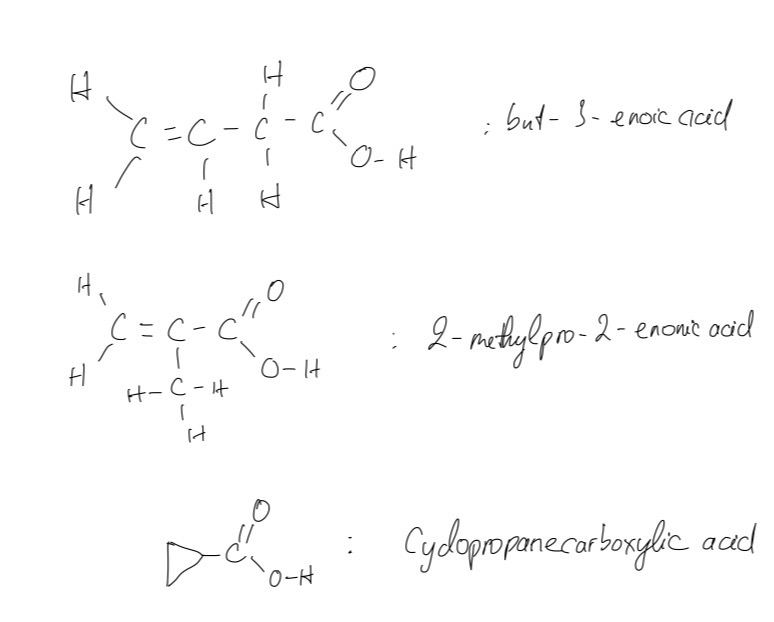
Question 6
Z is a saturated hydrocarbon that is an isomer of pentene. The molecular formula of Z matches the general formula of the homologous series of alkenes.
Draw the skeletal formula of Z.
Question 7
What is the identity of the functional group that is ringed in the molecule shown below?

A. Aldehyde
B. Ketone
C. Carboxylic acid
D. Alkene
The answer is B
A is incorrect because the carbon in the C=O of an aldehyde is bonded to a hydrogen and another carbon
C is incorrect because carboxylic acid has a -COOH functional group
D is incorrect because an alkene has a C=C functional group
Question 8
What is the IUPAC name of the following molecule?

A. 2-chloro-3-methylpentane
B. 3-methyl-4-chloropentane
C. 2-ethyl-3-chlorobutane
D. 2-chloro-3-ethylbutane
The answer is A
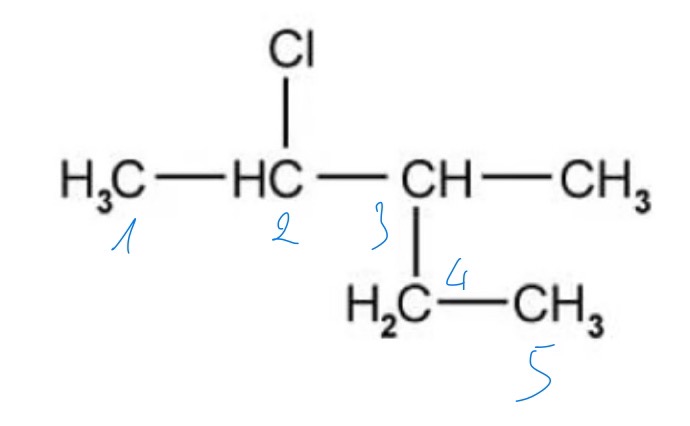
The naming rules begin with the longest carbon chain then counting the side groups and designate their position with the lowest number possible along the carbon chain
The -CH3 is a methyl group
The -Cl is a chloro group
B is incorrect because the numbers do not give the lowest combinations of the positions of the side groups along the chain
C and D are incorrect because the longest chain is 5 not 4
Question 9
Which of the following molecules are hydrocarbons?

A. 1 and 2
B. 1, 2, 3 and 4
C. 1, 2 and 3
D. 4 only
The answer is A
A hydrocarbon is a molecule containing only carbon and hydrogen
Molecules 3 and 4 contain another elements except carbon and hydrogen
Question 10
What is the correct formula for 1-fluro-3-methyl-2-butene?
| A. | Structural | `CH_2FCH_2 C(CH _3)CH_3` |
| B. | Empirical | C5H9F |
| C. | Displayed |  |
| D. | Skeletal |  |
The answer is B
A is incorrect because this it not a right formula due to having 10 H shown in the structure
C is incorrect because there is a methyl group on carbon 3
D is incorrect because this skeletal formula is not illustrating the fluorine and the double bond as well as the wrong branch of carbon
Question 1
A carbon compound P has the percentage composition 85.7 % carbon and 14.3 % hydrogen. Its relative molecular mass was found to be 56.
a.
i. Calculate its empirical formula.
ii. Calculate its molecular formula.
b. Write down the names and displayed formulae of all the non-cyclic isomers of compound P which have the following characteristics:
i. straight chain
ii. branched chain.
Question 2
He reacted ethane with chlorine in the presence of UV light by the following reaction:
`C _2H_6(g) + 2Cl_2(g) → C_2H_4Cl_2(l) + 2HCl(g)`
After doing this he found that 600 g of ethane gave 148.5 g of C2H4Cl2.
A. How many moles of ethane are there in 600 g?
B. How many moles of 1,2-dichloroethane would have been formed if the yield had been 100 %?
C. How many moles of 1,2-dichloroethane are there in 148.5 g?
D. Calculate the percentage yield of 1,2-dichloroethane.
Question 3
He reacted ethene with chlorine in the dark by the following reaction:
`C_2H_4(g) + Cl_2(g) -> C_2H_4Cl_2(l)`
In this reaction 140 g of ethene gave 396 g of C2H4Cl2.
A. Calculate the percentage yield for this reaction. Show your working.
B. There are isomers of the compound C2H4Cl2. Draw the displayed formulae of the isomers and name them.
C. Choose from redox, substitution, elimination, addition and hydrolysis to give the type of reaction for this reaction
Question 4
X can be manufactured by the oxidation of propene.
`CH_3CHCH_2 + O_2 → CH_2CHCHO + H_2O`
Name X and state the functional groups that are present.
Question 5
a. Crotonic acid, C4H6O2, is used in the manufacture of paints and adhesives.
State the empirical formula of crotonic acid.
b. The systematic name of crotonic acid is but-2-enoic acid.
Draw two displayed formulae for possible isomers of crotonic acid that retain the carboxylic acid functional group.
Question 6
Z is a saturated hydrocarbon that is an isomer of pentene. The molecular formula of Z matches the general formula of the homologous series of alkenes.
Draw the skeletal formula of Z.
Question 7
What is the identity of the functional group that is ringed in the molecule shown below?

A. Aldehyde
B. Ketone
C. Carboxylic acid
D. Alkene
Question 8
What is the IUPAC name of the following molecule?

A. 2-chloro-3-methylpentane
B. 3-methyl-4-chloropentane
C. 2-ethyl-3-chlorobutane
D. 2-chloro-3-ethylbutane
Question 9
Which of the following molecules are hydrocarbons?

A. 1 and 2
B. 1, 2, 3 and 4
C. 1, 2 and 3
D. 4 only
Question 10
What is the correct formula for 1-fluro-3-methyl-2-butene?
| A. | Structural | `CH_2FCH_2 C(CH _3)CH_3` |
| B. | Empirical | C5H9F |
| C. | Displayed |  |
| D. | Skeletal |  |